
What is the hybridization of chlorine atom in \[\text{Cl}{{\text{F}}_{\text{3}}}\]molecule?
Answer
592.5k+ views
Hint: Count the number of lone pairs and bond pairs of electrons present on the central chlorine atom.
Complete step by step answer:
Chlorine trifluoride contains a central chlorine atom which forms bonds with three fluorine atoms. Chlorine atom has 7 valence electrons with the valence shell electron configuration of \[3{{s}^{2}},3p_{x}^{2},3p_{y}^{1},3d\]. In a free gaseous (uncombined) chlorine atom, one unpaired electron is present. In chlorine trifluoride molecules, chlorine atoms have three bond pairs and two lone pairs. To form three bonds with three fluorine atoms, chlorine atoms should have three unpaired electrons. This is achieved by transfer of one electron from \[3{{p}_{x}}\text{ or }3{{p}_{y}}\] orbital to 3d orbital. This is done by the hybridisation of one 3s, three 3p and one 3d orbital. Five atomic orbitals participate in the hybridisation to give five hybrid orbitals. The hybridisation is \[s{{p}^{3}}d\] hybridisation. Out of the five hybrid orbitals, two will contain two lone pairs of electrons and three hybrid orbitals will contain three unpaired electrons. These three hybrid orbitals containing three unpaired electrons will overlap with orbitals of fluorine to form three bonds.
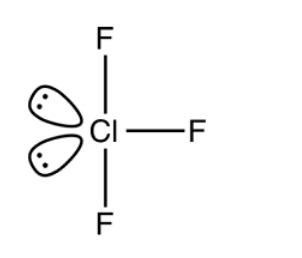
The electron pair geometry for three bond pairs of electrons and two lone pairs of electrons is trigonal bipyramidal. The molecular geometry is T-shaped with a bond angle of \[{{175}^{0}}\] . Two lone pairs of electrons occupy the equatorial positions in trigonal bipyramidal structure. The lone pair lone pair electron repulsions are greater than the bond pair lone pair electron repulsions and the bond pair bond pair electron repulsions. The molecular structure is as shown below:
Note:
Do not ignore the lone pairs of electrons while determining the type of hybridisation.
If you consider only 3 bond pairs of electrons, then \[s{{p}^{2}}\] hybridisation will give trigonal planar geometry. But when two lone pairs and three bond pairs are considered, \[s{{p}^{3}}d\] hybridisation gives electron pair geometry of trigonal bipyramidal shape and the molecule will have T-shaped geometry.
Complete step by step answer:
Chlorine trifluoride contains a central chlorine atom which forms bonds with three fluorine atoms. Chlorine atom has 7 valence electrons with the valence shell electron configuration of \[3{{s}^{2}},3p_{x}^{2},3p_{y}^{1},3d\]. In a free gaseous (uncombined) chlorine atom, one unpaired electron is present. In chlorine trifluoride molecules, chlorine atoms have three bond pairs and two lone pairs. To form three bonds with three fluorine atoms, chlorine atoms should have three unpaired electrons. This is achieved by transfer of one electron from \[3{{p}_{x}}\text{ or }3{{p}_{y}}\] orbital to 3d orbital. This is done by the hybridisation of one 3s, three 3p and one 3d orbital. Five atomic orbitals participate in the hybridisation to give five hybrid orbitals. The hybridisation is \[s{{p}^{3}}d\] hybridisation. Out of the five hybrid orbitals, two will contain two lone pairs of electrons and three hybrid orbitals will contain three unpaired electrons. These three hybrid orbitals containing three unpaired electrons will overlap with orbitals of fluorine to form three bonds.
The electron pair geometry for three bond pairs of electrons and two lone pairs of electrons is trigonal bipyramidal. The molecular geometry is T-shaped with a bond angle of \[{{175}^{0}}\] . Two lone pairs of electrons occupy the equatorial positions in trigonal bipyramidal structure. The lone pair lone pair electron repulsions are greater than the bond pair lone pair electron repulsions and the bond pair bond pair electron repulsions. The molecular structure is as shown below:

Note:
Do not ignore the lone pairs of electrons while determining the type of hybridisation.
If you consider only 3 bond pairs of electrons, then \[s{{p}^{2}}\] hybridisation will give trigonal planar geometry. But when two lone pairs and three bond pairs are considered, \[s{{p}^{3}}d\] hybridisation gives electron pair geometry of trigonal bipyramidal shape and the molecule will have T-shaped geometry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




