
What is the hybridization of the central atom of $SiO_2$?
A.\[sp\]
B.\[s{p^2}\]
C.\[s{p^3}\]
D.\[s{p^3}d\]
Answer
492.9k+ views
Hint: Chemical reactions are of many types, they are decomposition reaction, combination reaction, single-displacement reaction, double-displacement reaction and combustion reaction. All these reactions are carried out in different ways and give different products. To answer the above question, we will first state the type of reaction and then look at how those reactions undergo.
Complete answer:
We have to find hybridization of the compound, $SiO_2$ Before answering this let us look at different types of hybridizations.
We will learn about \[sp,s{p^2},s{p^3},s{p^3}d,s{p^3}{d^2},s{p^3}{d^3}\].
In \[sp\]hybridization: the mixing of 1 s-orbital and 1 p-orbital.
In \[s{p^2}\]hybridization: there is mixing of 1 s-orbital and 2-p orbitals.
In \[s{p^3}\]hybridization: there is mixing of 1 s-orbital and 3-p orbitals.
In \[s{p^3}d\]hybridization: there is mixing of 1 s-orbital, 3 p-orbitals and 1 d-orbital.
In \[s{p^3}{d^2}\] hybridization: there is mixing of 1 s-orbital, 3 p-orbitals and 2 d-orbitals.
In \[s{p^3}{d^3}\] hybridization: there is mixing of 1 s-orbital, 3 p-orbitals and 3 d-orbitals.
Let us look at the structure of SiO2, it is similar to the structure of CO2.
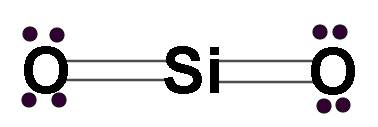
This compound has 2 lone pairs on each oxygen atom and 4 bonds in total.
There is a formula for calculating the hybridization it is,
Hybridization number= lone pairs on central atom + number of atoms attached to central metal atom.
Looking at the diagram we see that the central metal atom has no lone pair of electrons. And there are 2 atoms attached to the central metal atom.
Hence the hybridization number= 0+2=2
As the hybridization number is 2 its hybridization will be \[sp\].
Hence the correct answer to this question is option A.
Note:
There is another way of finding hybridization: it is the number of lone pairs + number of sigma bonds in the compound. Look at the table to determine the hybridization:
Like in this compound there are 2 sigma bonds and no lone pairs hence the hybridization will be \[sp\].
Complete answer:
We have to find hybridization of the compound, $SiO_2$ Before answering this let us look at different types of hybridizations.
We will learn about \[sp,s{p^2},s{p^3},s{p^3}d,s{p^3}{d^2},s{p^3}{d^3}\].
In \[sp\]hybridization: the mixing of 1 s-orbital and 1 p-orbital.
In \[s{p^2}\]hybridization: there is mixing of 1 s-orbital and 2-p orbitals.
In \[s{p^3}\]hybridization: there is mixing of 1 s-orbital and 3-p orbitals.
In \[s{p^3}d\]hybridization: there is mixing of 1 s-orbital, 3 p-orbitals and 1 d-orbital.
In \[s{p^3}{d^2}\] hybridization: there is mixing of 1 s-orbital, 3 p-orbitals and 2 d-orbitals.
In \[s{p^3}{d^3}\] hybridization: there is mixing of 1 s-orbital, 3 p-orbitals and 3 d-orbitals.
Let us look at the structure of SiO2, it is similar to the structure of CO2.
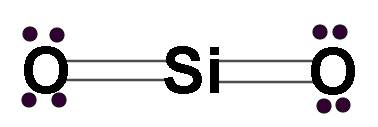
This compound has 2 lone pairs on each oxygen atom and 4 bonds in total.
There is a formula for calculating the hybridization it is,
Hybridization number= lone pairs on central atom + number of atoms attached to central metal atom.
Looking at the diagram we see that the central metal atom has no lone pair of electrons. And there are 2 atoms attached to the central metal atom.
Hence the hybridization number= 0+2=2
As the hybridization number is 2 its hybridization will be \[sp\].
Hence the correct answer to this question is option A.
Note:
There is another way of finding hybridization: it is the number of lone pairs + number of sigma bonds in the compound. Look at the table to determine the hybridization:
| Lone pairs+sigma bonds | hybridization |
| 2 | \[sp\] |
| 3 | \[s{p^2}\] |
| 4 | \[s{p^3}\] |
Like in this compound there are 2 sigma bonds and no lone pairs hence the hybridization will be \[sp\].
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




