
Hydroboration oxidation and acid hydration will yield the same product in case of:
A.
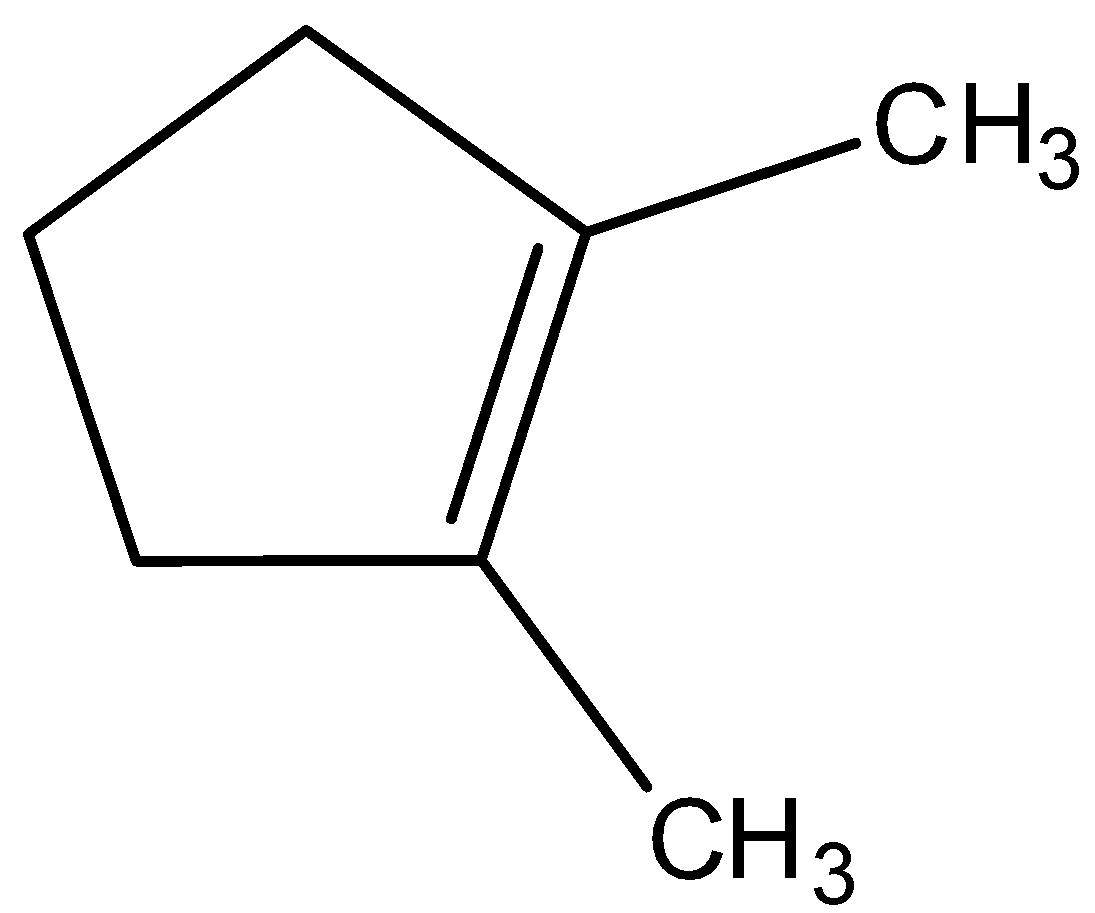
B.
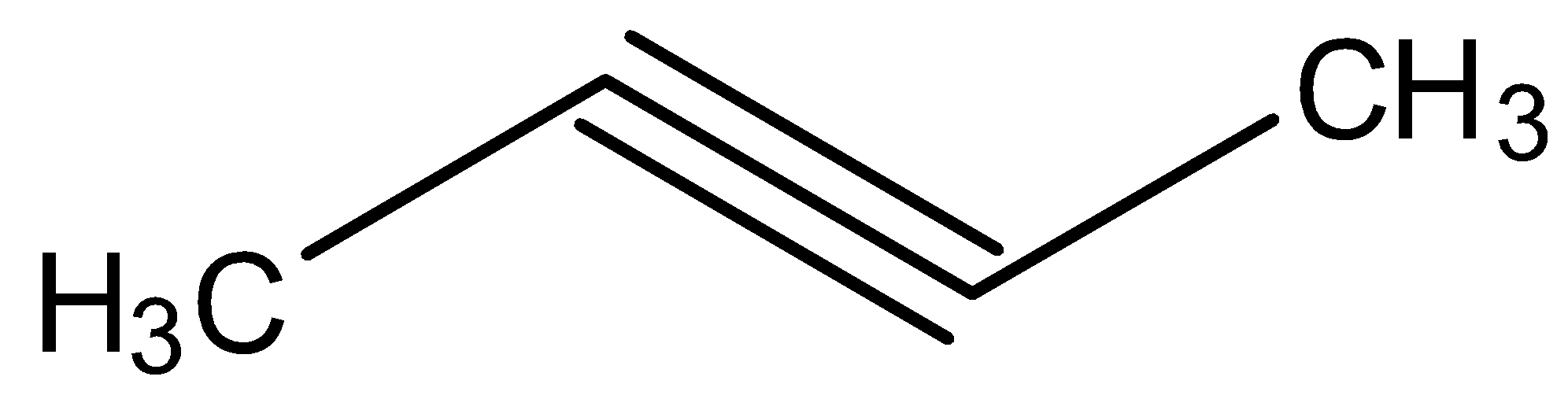
C.
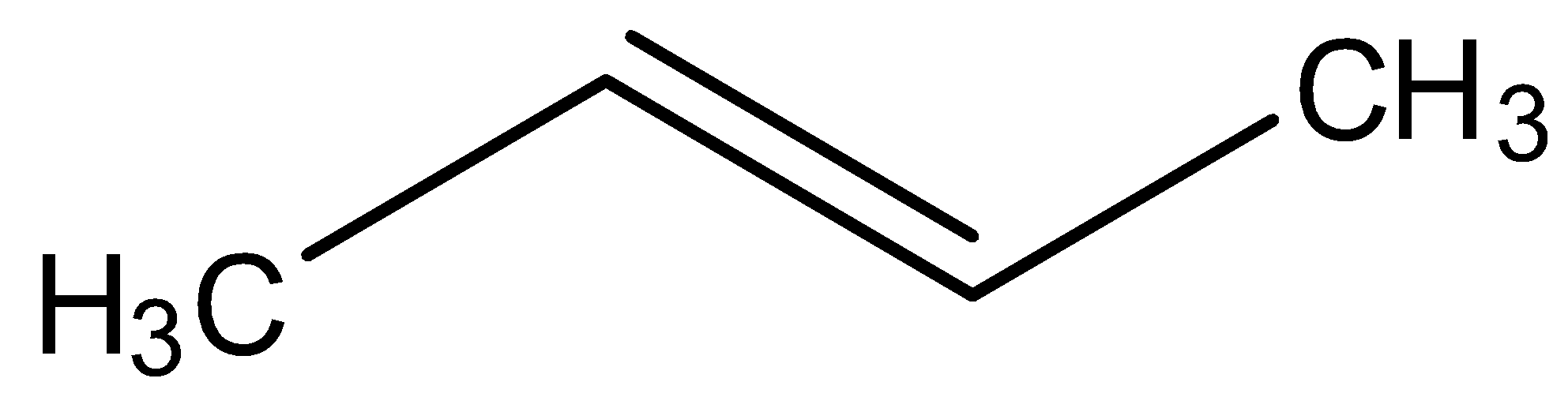
D.
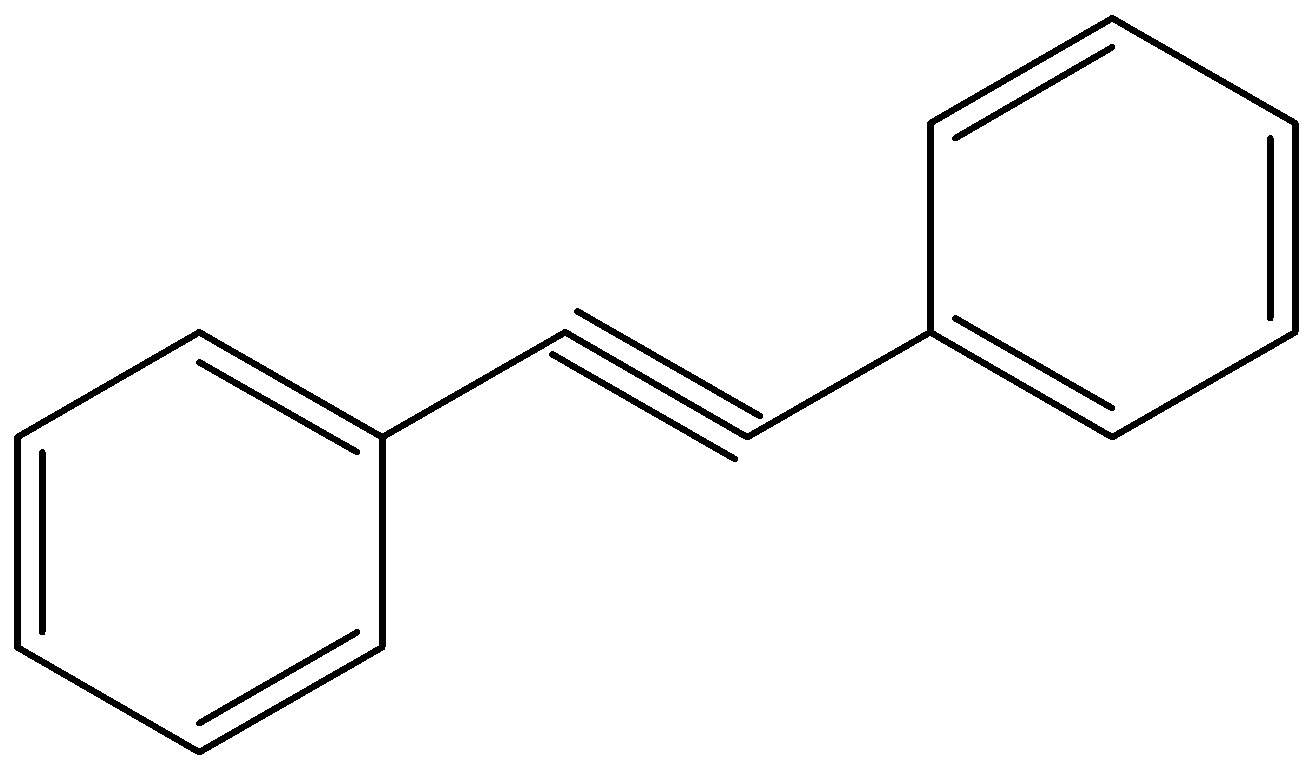
This question has multiple correct answers




Answer
592.8k+ views
Hint: Think about the mechanisms of both the reactions. Both the reactions involve the oxidation of an unsaturated bond and the formation of an alcohol. Remember that in the mechanisms of both the processes are different.
Complete step by step answer:
First, let us look at the hydroboration oxidation reaction.
- For alkenes:
This is a two-step reaction that converts an alkene into an alcohol. This is done when the unsaturated bond undergoes hydration. The reaction mechanism follows the anti-Markovnikov rule where the hydroxyl group attaches itself to the less substituted carbon atom.
In this reaction, 1 mole of an alkene combines with 1 mole of borane and 1 mole of peroxide in the presence of sodium hydroxide to give 1 mole of an alcohol and 1 mole of hydroxyborane.
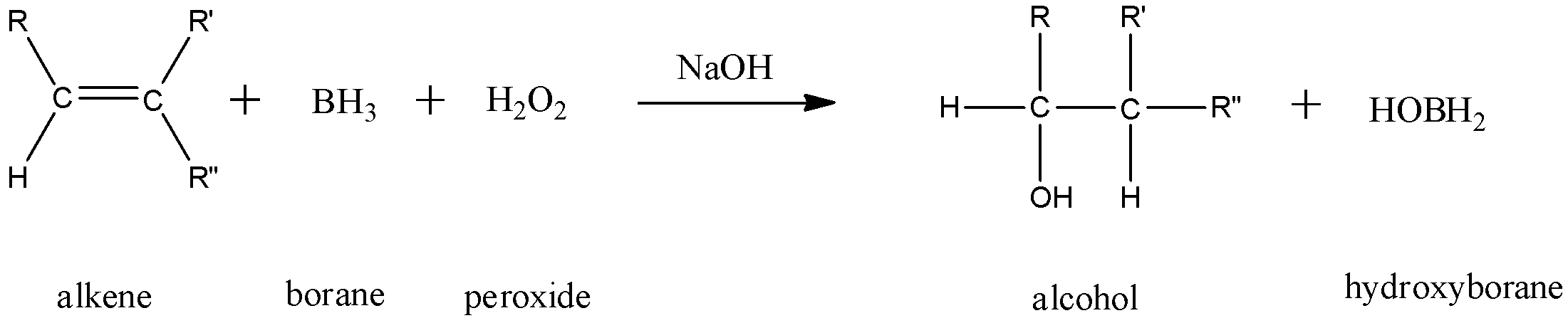
Here, we can see that the hydroxyl group has attached itself to the less substituted carbon atom.
- For alkynes:
The first step is the same as we have seen the hydroboration oxidation of alkenes. Instead of alcohol, an enol is formed. This enol then undergoes tautomerization to form an aldehyde or a ketone.
Now let us see the acid hydrolysis.
- For alkenes:
This is a reaction where the double bond on a hydrocarbon is hydrated. This reaction follows the Markovnikov’s rule where the hydroxyl ion will attach itself to the more substituted carbon atom.
In this reaction, 1 mole of an alkene combines with 1 mole of water in the presence of an acid to give 1 mole of an alcohol.
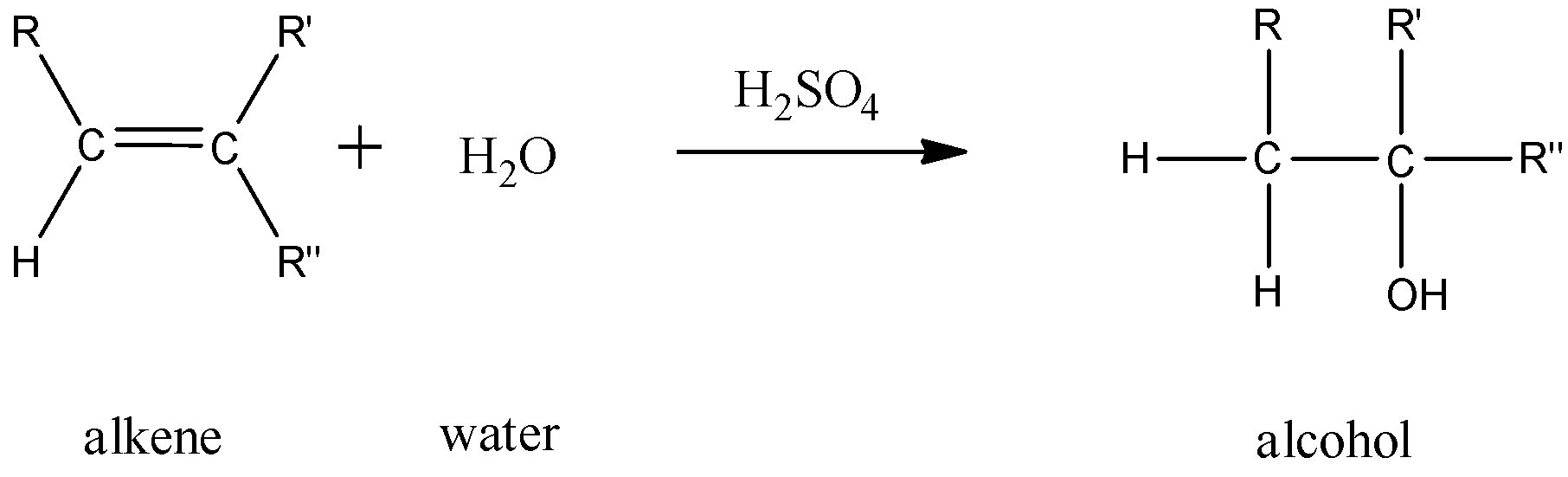
- For alkynes:
The first step of the reaction takes place in the same way as we have seen in alkenes. But, instead of alcohol, an enol is formed. This enol then undergoes tautomerization and forms an aldehyde or a ketone.
Looking at all the options given to us, we can deduce that all the points of unsaturation are symmetrical in nature. This means that the carbons present at both ends of the unsaturated bonds are equally substituted. Thus, the reason that these two processes are marked as different becomes null as the Markovnikov or anti-Markovnikov rule cannot be implemented.
Hence, the products formed by the compounds by both hydroboration oxidation and acid hydrolysis will be the same for all the starting compounds.
All the options are correct answers.
Note: Remember the Markovnikov rule, the negative part of the reagent will attach itself to the carbon which is substituted more i.e. which has less hydrogen atoms attached to it. The anti-Markovnikov rule is also called the peroxide effect will give the exact opposite result in presence of a peroxide.
Complete step by step answer:
First, let us look at the hydroboration oxidation reaction.
- For alkenes:
This is a two-step reaction that converts an alkene into an alcohol. This is done when the unsaturated bond undergoes hydration. The reaction mechanism follows the anti-Markovnikov rule where the hydroxyl group attaches itself to the less substituted carbon atom.
In this reaction, 1 mole of an alkene combines with 1 mole of borane and 1 mole of peroxide in the presence of sodium hydroxide to give 1 mole of an alcohol and 1 mole of hydroxyborane.

Here, we can see that the hydroxyl group has attached itself to the less substituted carbon atom.
- For alkynes:
The first step is the same as we have seen the hydroboration oxidation of alkenes. Instead of alcohol, an enol is formed. This enol then undergoes tautomerization to form an aldehyde or a ketone.
Now let us see the acid hydrolysis.
- For alkenes:
This is a reaction where the double bond on a hydrocarbon is hydrated. This reaction follows the Markovnikov’s rule where the hydroxyl ion will attach itself to the more substituted carbon atom.
In this reaction, 1 mole of an alkene combines with 1 mole of water in the presence of an acid to give 1 mole of an alcohol.

- For alkynes:
The first step of the reaction takes place in the same way as we have seen in alkenes. But, instead of alcohol, an enol is formed. This enol then undergoes tautomerization and forms an aldehyde or a ketone.
Looking at all the options given to us, we can deduce that all the points of unsaturation are symmetrical in nature. This means that the carbons present at both ends of the unsaturated bonds are equally substituted. Thus, the reason that these two processes are marked as different becomes null as the Markovnikov or anti-Markovnikov rule cannot be implemented.
Hence, the products formed by the compounds by both hydroboration oxidation and acid hydrolysis will be the same for all the starting compounds.
All the options are correct answers.
Note: Remember the Markovnikov rule, the negative part of the reagent will attach itself to the carbon which is substituted more i.e. which has less hydrogen atoms attached to it. The anti-Markovnikov rule is also called the peroxide effect will give the exact opposite result in presence of a peroxide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




