
Hydrogen bonding is maximum in:
(A) Ethanol
(B) Diethyl ether
(C) Ethyl chloride
(D) Trimethylamine
Answer
519.9k+ views
Hint: Hydrogen bonding is a special type of dipole-dipole interaction between a hydrogen atom and an highly electronegative element like O and N. Hydrogen bonding is maximum in those compounds which have less sterically hindered H atoms.
Complete step by step answer:
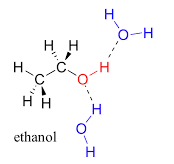
A) In ethanol, the OH group is present. This means an H atom is attached to an O atom. The electronegativity of an O atom is much greater than an H atom because of this the H atom will get a partial positive charge and the O atom will get a partially negative charge. This is known as a dipole. Hence dipole-dipole interactions will get generated in the molecule and attractive forces will be produced among the molecules of ethanol.
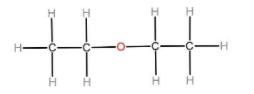
B) There is no hydrogen bonding between diethyl ether molecules because there is no hydrogen atom attached to an electronegative atom like oxygen or nitrogen. So diethyl ether does not show hydrogen bonding.
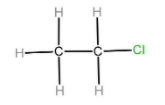
C) Chlorine is less electronegative as compared to oxygen and nitrogen so it does not show hydrogen bonding. So, ethanol has more hydrogen bonding than ethyl chloride.
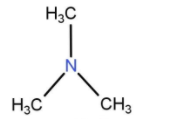
D) In trimethylamine, the nitrogen atom is not attached to any hydrogen atoms. So, it will not show any hydrogen bonding.
According to above mentioned points, we can conclude that out of the given options ethanol will have maximum hydrogen bonding.
So, the correct option is (A).
Additional Information:
Properties of hydrogen bonding :
i) Solubility: Because of the hydrogen bonding between water and alcohol molecules, lower alcohols are soluble in water.
ii) Volatility: Compounds having hydrogen bonding between different molecules have a higher boiling point and a higher boiling point means the compound is less volatile.
iii) Viscosity and surface tension: The substances which contain hydrogen bonding exist as an associated molecule. They have higher viscosity and high surface tension.
Note: In alcohols the extent of hydrogen bonding decreases with increase in molecular mass. This is why lower alcohols are highly soluble in water than higher alcohols. Hydrogen bonding is the reason for the physical state of alcohol. Maximum hydrogen bonding in alcohols means liquid state.
Complete step by step answer:
A) In ethanol, the OH group is present. This means an H atom is attached to an O atom. The electronegativity of an O atom is much greater than an H atom because of this the H atom will get a partial positive charge and the O atom will get a partially negative charge. This is known as a dipole. Hence dipole-dipole interactions will get generated in the molecule and attractive forces will be produced among the molecules of ethanol.

B) There is no hydrogen bonding between diethyl ether molecules because there is no hydrogen atom attached to an electronegative atom like oxygen or nitrogen. So diethyl ether does not show hydrogen bonding.

C) Chlorine is less electronegative as compared to oxygen and nitrogen so it does not show hydrogen bonding. So, ethanol has more hydrogen bonding than ethyl chloride.

D) In trimethylamine, the nitrogen atom is not attached to any hydrogen atoms. So, it will not show any hydrogen bonding.

According to above mentioned points, we can conclude that out of the given options ethanol will have maximum hydrogen bonding.
So, the correct option is (A).
Additional Information:
Properties of hydrogen bonding :
i) Solubility: Because of the hydrogen bonding between water and alcohol molecules, lower alcohols are soluble in water.
ii) Volatility: Compounds having hydrogen bonding between different molecules have a higher boiling point and a higher boiling point means the compound is less volatile.
iii) Viscosity and surface tension: The substances which contain hydrogen bonding exist as an associated molecule. They have higher viscosity and high surface tension.
Note: In alcohols the extent of hydrogen bonding decreases with increase in molecular mass. This is why lower alcohols are highly soluble in water than higher alcohols. Hydrogen bonding is the reason for the physical state of alcohol. Maximum hydrogen bonding in alcohols means liquid state.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




