
Hydrogen peroxide is prepared in the laboratory by:
A.Adding $Mn{O_2}$ to dilute ${H_2}S{O_4}$
B.Passing $C{O_2}$ into a paste in cold water
C.Adding $Pb{O_2}$ to an acidified $KMn{O_4}$
D.Adding $N{a_2}{O_2}$to cold water
Answer
232.8k+ views
Hint: To answer this question, you should recall the concept of preparation of hydrogen peroxide. It contains oxygen in an intermediate oxidation state and can thus, act as both oxidising and reducing reagent.
Complete step by step answer:
We know that peroxide is a compound which has two oxygen atoms bonded together. And Hydrogen Peroxide is the simplest peroxide. The chemical formula for hydrogen peroxide is ${H_2}{O_2}$. The structure of the molecule can be drawn as:
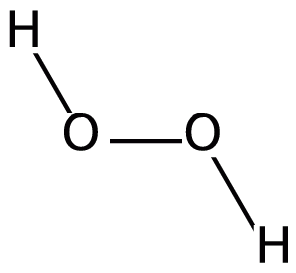
Now we know the methods of preparation of ${H_2}{O_2}$ so let us analyse each of the options systematically:
A.A balanced chemical reaction for this process can be written as \[2Mn{O_2}\; + {\text{ }}2{H_2}S{O_4} \to \;\;2MnS{O_4}\; + {\text{ }}{O_2}\; + {\text{ }}2{H_2}O\].
We can see that \[{H_2}{O_2}\]is not being formed in this reaction hence the given option is wrong and can be eliminated.
B.$C{O_2} + {H_2}O \to {H_2}C{O_3}$.
We can see that \[{H_2}{O_2}\]is not being formed in this reaction, thus the given option is wrong and can be eliminated.
C.\[5Pb{O_2} + 2M{n^{2 \oplus }} + 4{H^ \oplus } \to 5P{b^{2 \oplus }} + 2MnO{4^ - } + 2H2O\].
It is evident that \[{H_2}{O_2}\] is not being formed in this reaction, the given option is wrong and can be eliminated
D.\[N{a_2}{O_2}\left( s \right) + {H_2}S{O_4} \to N{a_2}S{O_4}\left( s \right) + {H_2}{O_2}\left( l \right)\].
We can see that here \[{H_2}{O_2}\]is being produced. This is indeed a preparation method of laboratory preparation of hydrogen peroxide by the action of ice-cold, dilute sulphuric acid on sodium peroxide or hydrated barium peroxide. A calculated quantity of sodium peroxide is added in small quantities to $20\% $ice-cold solution of sulphuric acid. Most of the sodium sulphate separates on cooling as crystals of \[N{a_2}S{O_4}.10{H_2}O\]. From this reaction, we obtain a \[30\% \] hydrogen peroxide yield containing a small amount of sodium sulphate.
Hence, we can conclude that the correct answer to this question is option D
Note: You must remember all the preparation methods of hydrogen peroxide which are most commonly used in the laboratories. Apart from the above reaction, the other important reaction for laboratory preparation of hydrogen peroxide is from barium peroxide: \[Ba{O_2}.8{H_2}O\; + {\text{ }}{H_2}S{O_{4\;}} \to {\text{ }}BaS{O_4}\; + {\text{ }}{H_2}{O_2}\; + {\text{ }}8{H_2}O\]
Complete step by step answer:
We know that peroxide is a compound which has two oxygen atoms bonded together. And Hydrogen Peroxide is the simplest peroxide. The chemical formula for hydrogen peroxide is ${H_2}{O_2}$. The structure of the molecule can be drawn as:

Now we know the methods of preparation of ${H_2}{O_2}$ so let us analyse each of the options systematically:
A.A balanced chemical reaction for this process can be written as \[2Mn{O_2}\; + {\text{ }}2{H_2}S{O_4} \to \;\;2MnS{O_4}\; + {\text{ }}{O_2}\; + {\text{ }}2{H_2}O\].
We can see that \[{H_2}{O_2}\]is not being formed in this reaction hence the given option is wrong and can be eliminated.
B.$C{O_2} + {H_2}O \to {H_2}C{O_3}$.
We can see that \[{H_2}{O_2}\]is not being formed in this reaction, thus the given option is wrong and can be eliminated.
C.\[5Pb{O_2} + 2M{n^{2 \oplus }} + 4{H^ \oplus } \to 5P{b^{2 \oplus }} + 2MnO{4^ - } + 2H2O\].
It is evident that \[{H_2}{O_2}\] is not being formed in this reaction, the given option is wrong and can be eliminated
D.\[N{a_2}{O_2}\left( s \right) + {H_2}S{O_4} \to N{a_2}S{O_4}\left( s \right) + {H_2}{O_2}\left( l \right)\].
We can see that here \[{H_2}{O_2}\]is being produced. This is indeed a preparation method of laboratory preparation of hydrogen peroxide by the action of ice-cold, dilute sulphuric acid on sodium peroxide or hydrated barium peroxide. A calculated quantity of sodium peroxide is added in small quantities to $20\% $ice-cold solution of sulphuric acid. Most of the sodium sulphate separates on cooling as crystals of \[N{a_2}S{O_4}.10{H_2}O\]. From this reaction, we obtain a \[30\% \] hydrogen peroxide yield containing a small amount of sodium sulphate.
Hence, we can conclude that the correct answer to this question is option D
Note: You must remember all the preparation methods of hydrogen peroxide which are most commonly used in the laboratories. Apart from the above reaction, the other important reaction for laboratory preparation of hydrogen peroxide is from barium peroxide: \[Ba{O_2}.8{H_2}O\; + {\text{ }}{H_2}S{O_{4\;}} \to {\text{ }}BaS{O_4}\; + {\text{ }}{H_2}{O_2}\; + {\text{ }}8{H_2}O\]
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




