
Hydrolysis of ozonide of 1-butene gives:
(a) ethylene only
(b) acetaldehyde and formaldehyde
(c) propionaldehyde and formaldehyde
(d) acetaldehyde only
Answer
573.6k+ views
Hint: Ozonolysis is the process by which the double bond in alkenes is converted to the single bond and structure thus, is called as ozonide which on hydrolysis in the presence of zinc dust give two compounds and each of the resultant compound consists of C=O bond in them. Now, solve it.
Complete step by step answer:
First let’s discuss what is an ozonide. Ozonide is formed when an alkene is made to react with the ozone molecule following hydrolysis. And ozone is a triatomic molecule of oxygen and it exists in the stratosphere about 20 km away from the surface of the earth.
When Alkene is reacted with the ozone molecule, the double bond present in the alkene between the C=C is converted to single C-C single and results in the formation of structure called as the ozonide and when it is made to undergo hydrolysis in the presence of Zn dust , it results in the formation of two compounds and each of them contains one carbon-oxygen bond. Consider the ozonolysis reaction of the 1-butene. The reaction occurs as:
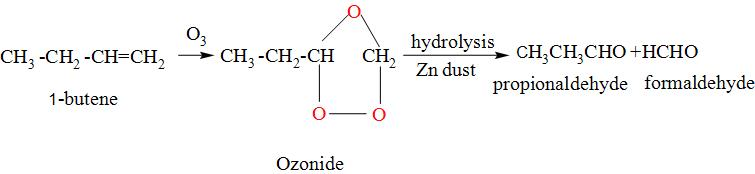
So, thus the ozonolysis of 1-butene on hydrolysis gives propionaldehyde and formaldehyde.
Hence, option(c) is correct.
Note: The hydrolysis product of ozonolysis depends on the presence or absence of hydrogen atom along with the carbon atom that is forming the ozonide. If the hydrogen is attached to the carbon atom, then the hydrolysis are aldehydes and if there is no hydrogen atom attached then the hydrolysis product is ketone. In 1-butene, the hydrogen atoms are present along with carbon atoms and thus, on hydrolysis, it gives aldehyde.
Complete step by step answer:
First let’s discuss what is an ozonide. Ozonide is formed when an alkene is made to react with the ozone molecule following hydrolysis. And ozone is a triatomic molecule of oxygen and it exists in the stratosphere about 20 km away from the surface of the earth.
When Alkene is reacted with the ozone molecule, the double bond present in the alkene between the C=C is converted to single C-C single and results in the formation of structure called as the ozonide and when it is made to undergo hydrolysis in the presence of Zn dust , it results in the formation of two compounds and each of them contains one carbon-oxygen bond. Consider the ozonolysis reaction of the 1-butene. The reaction occurs as:

So, thus the ozonolysis of 1-butene on hydrolysis gives propionaldehyde and formaldehyde.
Hence, option(c) is correct.
Note: The hydrolysis product of ozonolysis depends on the presence or absence of hydrogen atom along with the carbon atom that is forming the ozonide. If the hydrogen is attached to the carbon atom, then the hydrolysis are aldehydes and if there is no hydrogen atom attached then the hydrolysis product is ketone. In 1-butene, the hydrogen atoms are present along with carbon atoms and thus, on hydrolysis, it gives aldehyde.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




