
Hyper conjugation is?
(A) $\sigma -\pi $ Conjugation
(B) Noticed due to delocalization of $\sigma -\pi $
(C) no bond response
(D) all the above
Answer
591.9k+ views
Hint: When an alkyl group is attached to an unsaturated system such as double bond or benzene ring, the order of inductive effect is actually reversed. This effect is called hyperconjugation effect, which involves delocalization of \[\sigma \] -electrons through overlapping p-orbitals of double bond with \[\sigma \]-bond of the adjacent single bond.
Complete answer:
Hyper conjugation is a permanent electron displacement effect. This is also called “No bond resonance”.
More alpha C-H bond, more will be the no bond resonating structure, which will be more stability radical due to hyper conjugation.
Consider, the presence of $\alpha -H$ with respect to double bond, triple bond carbon containing positive charge in carbonium ion or unpaired electron with $\sigma -\pi $ conjugation.
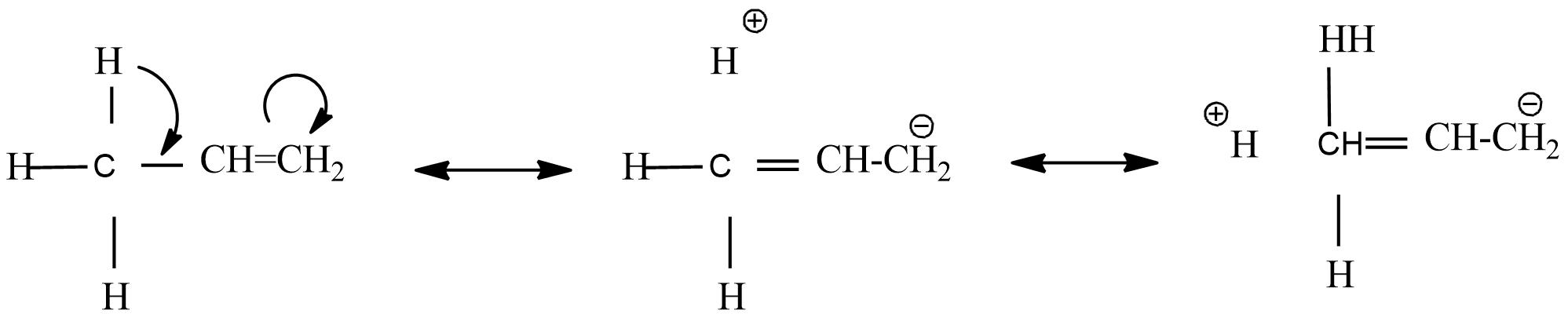
Hyper conjugation is the delocalization of $\sigma $ and $\pi $ bonds. From the above resonance structure, found that it ends in addition to bonds where there is no bond between C-H. Hence, there is no bond resonance.
So, all options are correct.
So, the correct answer is “Option D”.
Note: The inductive effect and hyperconjugation effect, both are quite opposite. Because Inductive effect through sigma bonds, while hyperconjugation is through total transfer charge. Hyper conjugation is very much useful in explaining the physical and chemical properties of organic molecules, like directive influence of alkyl group, shortening of carbon-carbon single bonds adjacent to multiple bonds, stability of carbocations and free radicals and relative stability of alkenes.
Complete answer:
Hyper conjugation is a permanent electron displacement effect. This is also called “No bond resonance”.
More alpha C-H bond, more will be the no bond resonating structure, which will be more stability radical due to hyper conjugation.
Consider, the presence of $\alpha -H$ with respect to double bond, triple bond carbon containing positive charge in carbonium ion or unpaired electron with $\sigma -\pi $ conjugation.

Hyper conjugation is the delocalization of $\sigma $ and $\pi $ bonds. From the above resonance structure, found that it ends in addition to bonds where there is no bond between C-H. Hence, there is no bond resonance.
So, all options are correct.
So, the correct answer is “Option D”.
Note: The inductive effect and hyperconjugation effect, both are quite opposite. Because Inductive effect through sigma bonds, while hyperconjugation is through total transfer charge. Hyper conjugation is very much useful in explaining the physical and chemical properties of organic molecules, like directive influence of alkyl group, shortening of carbon-carbon single bonds adjacent to multiple bonds, stability of carbocations and free radicals and relative stability of alkenes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




