
I. The formulas of ethane, ethyne and ethene are ${C_2}{H_6},{C_2}{H_4}$ and ${C_2}{H_2}$ . Because:
II. Alkanes contain only $C - C$ and $C - H$ bonds, alkenes may contain triple bonds between carbon atoms and alkynes may contain double bonds between carbon atoms.
A. Statement I is true, Statement II is true.
B. Statement I is true, Statement II is false.
C. Statement I is false, Statement I is true.
D. Statement I is false, Statement II is false.
E. Statement I is true, Statement II is true and is a correct explanation of the phenomena described in I.
Answer
583.2k+ views
Hint: Ethane is a saturated hydrocarbon with no multiple bonds in it. Ethene and ethyne are unsaturated hydrocarbons with double and triple bonds in them, respectively. The more the number of bonds increases between two adjacent carbon atoms, the more the decrease in the number of hydrogen atoms attached to the carbon will be.
Complete step by step answer:
The ethane is the second compound found in the series of alkanes and consists of two carbon atoms. The alkanes have a general chemical formula of ${C_n}{H_{2n + 2}}$ . When the value of $n = 2$ , then the chemical formula becomes ${C_2}{H_6}$ and the structure is named as ethane according to IUPAC. Ethane in total consists of one $C - C$ bond and six $C - H$ bonds. The structure of ethane is as follows:
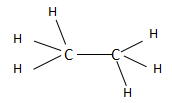
The ethene is the first compound found in the series of alkenes and consists of two carbon atoms. The alkenes have a general chemical formula of ${C_n}{H_{2n}}$ . When the value of $n = 2$ , then the chemical formula becomes ${C_2}{H_4}$ and the structure is named as ethene according to IUPAC. Ethene in total consists of two $C - C$ bonds and four $C - H$ bonds. The structure of ethane is as follows:
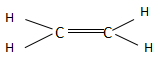
The ethyne is the first compound found in the series of alkenes and consists of two carbon atoms. The alkenes have a general chemical formula of ${C_n}{H_{2n - 2}}$ . When the value of $n = 2$ , then the chemical formula becomes ${C_2}{H_2}$ and the structure is named ethyne according to IUPAC. Ethyne in total consists of three $C - C$ bonds and two $C - H$ bonds. The structure of ethane is as follows:
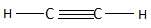
So, the correct answer is Option D .
Note:
The degree of saturation in the alkanes is equal to zero, in the alkenes, it is equal to one and in the alkynes, it is equal to two. The first bond formed between two carbon atoms is always a sigma bond and the bonds formed later, i.e. double and triple bonds are pi-bonds and are formed by parallel overlapping of orbitals and are generally weaker than the sigma overlapping. The order of bond strength in between ethane, ethene and ethyne is as follows:
${C_2}{H_2} > {C_2}{H_4} > {C_2}{H_6}$
Complete step by step answer:
The ethane is the second compound found in the series of alkanes and consists of two carbon atoms. The alkanes have a general chemical formula of ${C_n}{H_{2n + 2}}$ . When the value of $n = 2$ , then the chemical formula becomes ${C_2}{H_6}$ and the structure is named as ethane according to IUPAC. Ethane in total consists of one $C - C$ bond and six $C - H$ bonds. The structure of ethane is as follows:

The ethene is the first compound found in the series of alkenes and consists of two carbon atoms. The alkenes have a general chemical formula of ${C_n}{H_{2n}}$ . When the value of $n = 2$ , then the chemical formula becomes ${C_2}{H_4}$ and the structure is named as ethene according to IUPAC. Ethene in total consists of two $C - C$ bonds and four $C - H$ bonds. The structure of ethane is as follows:
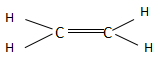
The ethyne is the first compound found in the series of alkenes and consists of two carbon atoms. The alkenes have a general chemical formula of ${C_n}{H_{2n - 2}}$ . When the value of $n = 2$ , then the chemical formula becomes ${C_2}{H_2}$ and the structure is named ethyne according to IUPAC. Ethyne in total consists of three $C - C$ bonds and two $C - H$ bonds. The structure of ethane is as follows:

So, the correct answer is Option D .
Note:
The degree of saturation in the alkanes is equal to zero, in the alkenes, it is equal to one and in the alkynes, it is equal to two. The first bond formed between two carbon atoms is always a sigma bond and the bonds formed later, i.e. double and triple bonds are pi-bonds and are formed by parallel overlapping of orbitals and are generally weaker than the sigma overlapping. The order of bond strength in between ethane, ethene and ethyne is as follows:
${C_2}{H_2} > {C_2}{H_4} > {C_2}{H_6}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




