
Identify compounds in which the central atom has incomplete octet.
\[B{{F}_{3}},AlC{{l}_{3}},GaC{{l}_{2}},MgC{{l}_{2}},BeC{{l}_{2}}\]
Answer
582.9k+ views
Hint: Incomplete octet means, less than 8 electrons in the central atom after bond forming between the central atom and the surrounding atom and thus it makes it unstable. It can be checked by drawing the Lewis dot structure of each compound.
Complete step by step solution:
From your chemistry lessons you have learned about the octet rule. It states that any compound will tend to combine in such a way that each atom in the compound will have eight electrons in their valence electron, and thus they become stable.
This rule is applicable to main group elements mainly nitrogen , oxygen, carbon and halogen but it is also applied on magnesium and sodium.
Now, let us check which among the following compounds given follows octet rule, to check the no. of valence electrons on each atom you write their electron configuration and draw a Lewis dot structure. Valence electrons are those electrons which are found in the outermost shell of an atom and participate in formation of chemical bonds with other atoms.
(A) $B{{F}_{3}}$
In this compound the central atom is Boron and it is surrounded by three fluorine atoms. So, to draw a Lewis dot structure firstly we have to find the no. of valence electrons in each atom.
No. of valence electrons in boron (atomic no. 5) = 3
No. of valence electrons in Fluorine (atomic no. 9) = 7
So, the Lewis structure will be,
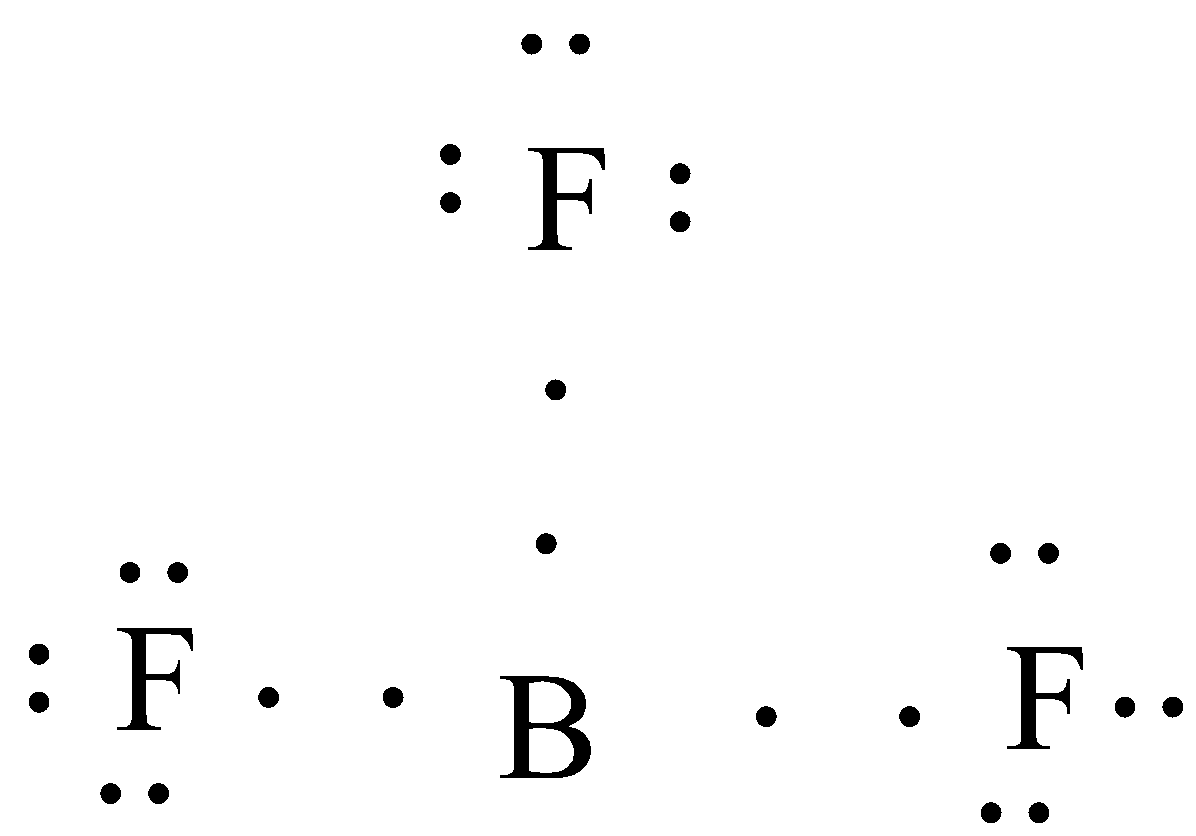
Here, you can see that each fluorine is sharing one electron with the central bromine atom, but the octet of bromine is not attained because it will contain only six valence electrons after the sharing .
Thus in $B{{F}_{3}}$ central atoms will have incomplete octets.
(B) $AlC{{l}_{3}}$
Here the central atom will be aluminium surrounded with three chlorine atom
No. of valence electrons in aluminium (atomic no. 13) = 3
No. of valence electrons in Chlorine (atomic no. 17) = 7
So, the Lewis dot structure is,
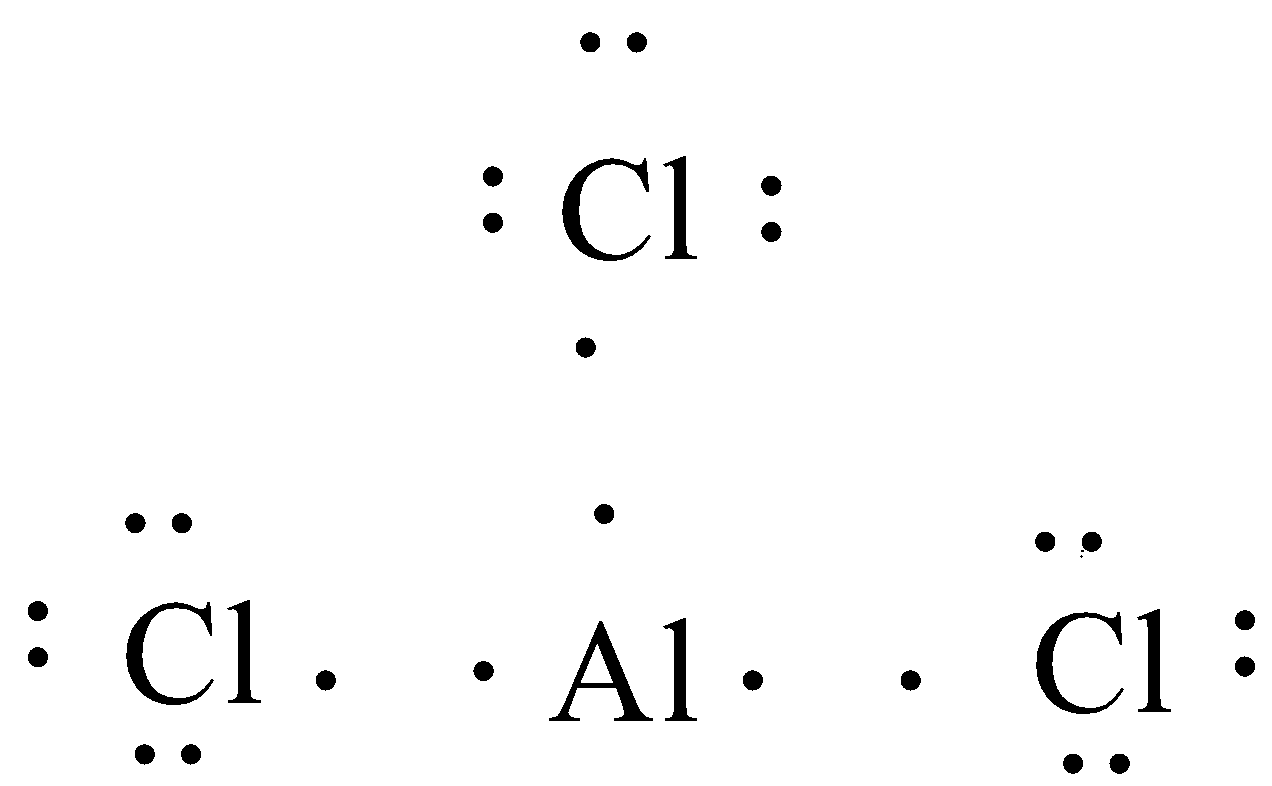
In this case also the central atom is not having eight complete electrons.
Thus in $AlC{{l}_{3}}$ central atoms will have incomplete octets.
\[BeC{{l}_{2}}\] will also have incomplete octet in its central atom
In the other two compounds the central atom will have a complete octet in them.
Thus the correct answer will be $B{{F}_{3}}$, $AlC{{l}_{3}}$and $BeC{{l}_{2}}$.
Note: Bromine and aluminium are the exception of octet rule because even after having only six electrons in their valence electron they were efficient as other compound works. Back bonding is seen in $B{{F}_{3}}$. $AlC{{l}_{3}}$ and $BeC{{l}_{2}}$forms dimer to complete their octet.
Complete step by step solution:
From your chemistry lessons you have learned about the octet rule. It states that any compound will tend to combine in such a way that each atom in the compound will have eight electrons in their valence electron, and thus they become stable.
This rule is applicable to main group elements mainly nitrogen , oxygen, carbon and halogen but it is also applied on magnesium and sodium.
Now, let us check which among the following compounds given follows octet rule, to check the no. of valence electrons on each atom you write their electron configuration and draw a Lewis dot structure. Valence electrons are those electrons which are found in the outermost shell of an atom and participate in formation of chemical bonds with other atoms.
(A) $B{{F}_{3}}$
In this compound the central atom is Boron and it is surrounded by three fluorine atoms. So, to draw a Lewis dot structure firstly we have to find the no. of valence electrons in each atom.
No. of valence electrons in boron (atomic no. 5) = 3
No. of valence electrons in Fluorine (atomic no. 9) = 7
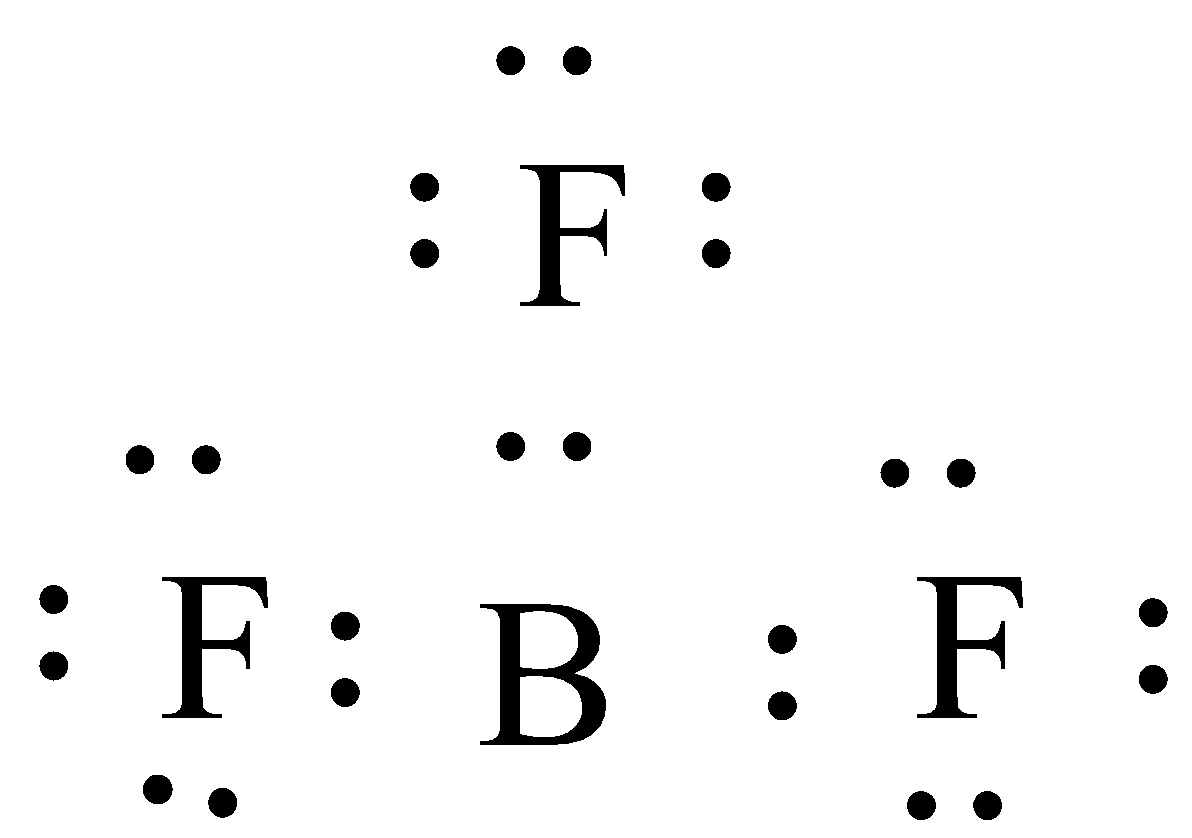
So, the Lewis structure will be,


Here, you can see that each fluorine is sharing one electron with the central bromine atom, but the octet of bromine is not attained because it will contain only six valence electrons after the sharing .
Thus in $B{{F}_{3}}$ central atoms will have incomplete octets.
(B) $AlC{{l}_{3}}$
Here the central atom will be aluminium surrounded with three chlorine atom
No. of valence electrons in aluminium (atomic no. 13) = 3
No. of valence electrons in Chlorine (atomic no. 17) = 7
So, the Lewis dot structure is,

In this case also the central atom is not having eight complete electrons.
Thus in $AlC{{l}_{3}}$ central atoms will have incomplete octets.
\[BeC{{l}_{2}}\] will also have incomplete octet in its central atom
In the other two compounds the central atom will have a complete octet in them.
Thus the correct answer will be $B{{F}_{3}}$, $AlC{{l}_{3}}$and $BeC{{l}_{2}}$.
Note: Bromine and aluminium are the exception of octet rule because even after having only six electrons in their valence electron they were efficient as other compound works. Back bonding is seen in $B{{F}_{3}}$. $AlC{{l}_{3}}$ and $BeC{{l}_{2}}$forms dimer to complete their octet.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




