
Identify most acidic hydrogen in given compound.
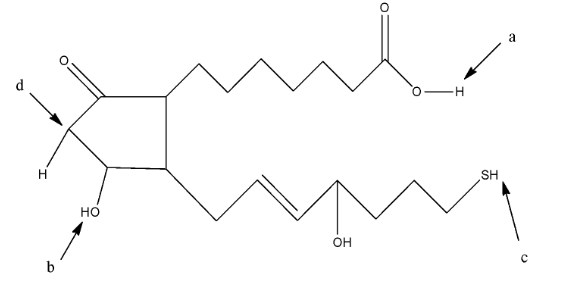
(A)- c
(B)- b
(C)- a
(D)- d

Answer
542.4k+ views
Hint:The acidic hydrogen means the hydrogen atom which will easily release from the compound and make the solution acidic. So, the hydrogen atom which will form a stable resonating structure after its removal will be the most acidic hydrogen atom.
Complete step-by-step answer:We know that the solution becomes acidic when there is the presence of hydrogen ions (${{H}^{+}}$) in the solution. The acidic hydrogen means the hydrogen atom which will easily release from the compound and make the solution acidic. The given compound the question is:
In this compound, we have to find the most acidic hydrogen. So, the hydrogen atom which will form a stable resonating structure after its removal will be the most acidic hydrogen atom. This is due to the fact that the resonance in the compound will make the compound stable.
The hydrogen atom attached to the acidic group will be the most acidic because the hydrogen atom is attached with very electronegative atom oxygen. In a, the hydrogen atom is attached with an oxygen atom which is further attached with a carbon atom having a double bond, so it is an acidic hydrogen. In b, the hydrogen atom is attached to the oxygen atom but it is not further attached to a carbon atom. In c, the hydrogen atom is attached to a sulfur atom. In d, the hydrogen atom is attached to a carbon atom.
So, the hydrogen atom at a position will be the most acidic hydrogen.
Therefore, the correct answer is an option (C)- a.
Note:When there is a double bond, the hydrogen atom is more acidic than the single bond because the s-character increases and the triple bond has more acidity as the s-character in the triple bond is the highest.
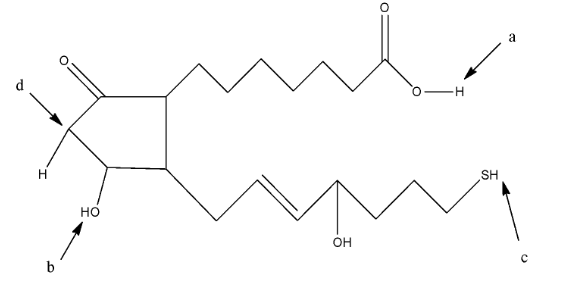
Complete step-by-step answer:We know that the solution becomes acidic when there is the presence of hydrogen ions (${{H}^{+}}$) in the solution. The acidic hydrogen means the hydrogen atom which will easily release from the compound and make the solution acidic. The given compound the question is:

In this compound, we have to find the most acidic hydrogen. So, the hydrogen atom which will form a stable resonating structure after its removal will be the most acidic hydrogen atom. This is due to the fact that the resonance in the compound will make the compound stable.
The hydrogen atom attached to the acidic group will be the most acidic because the hydrogen atom is attached with very electronegative atom oxygen. In a, the hydrogen atom is attached with an oxygen atom which is further attached with a carbon atom having a double bond, so it is an acidic hydrogen. In b, the hydrogen atom is attached to the oxygen atom but it is not further attached to a carbon atom. In c, the hydrogen atom is attached to a sulfur atom. In d, the hydrogen atom is attached to a carbon atom.
So, the hydrogen atom at a position will be the most acidic hydrogen.
Therefore, the correct answer is an option (C)- a.
Note:When there is a double bond, the hydrogen atom is more acidic than the single bond because the s-character increases and the triple bond has more acidity as the s-character in the triple bond is the highest.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




