
Identify the structure of propanoic anhydride.
(A) $ {\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CO}}} \right)_{\text{2}}}{\text{O}} $
(B) $ {\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}}} \right)_{\text{2}}}{\text{O}} $
(C) $ {\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CO}}} \right)_{\text{2}}}{\text{O}} $
(D) $ {\text{C}}{{\text{H}}_{\text{3}}}{\text{COOCOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}} $
Answer
533.4k+ views
Hint: An acid anhydride is a functional derivative of carboxylic acid. In organic chemistry, an acid anhydride contains the functional group $ {\text{R}}\left( {{\text{CO}}} \right){\text{O}}\left( {{\text{CO}}} \right){\text{R'}} $ where R and $ {\text{R'}} $ are two alkyl groups.
Complete Step By Step Answer:
When carboxylic acids are heated in the presence of a strong dehydrating agent such as phosphorus pentoxide or concentrated sulphuric acid, acid anhydrides are formed by the elimination of a molecule of water from two molecules of the acid. For example, ethanoic anhydride is obtained by the treatment of two molecules of ethanoic acid in presence of phosphorus pentoxide or concentrated sulphuric acid.
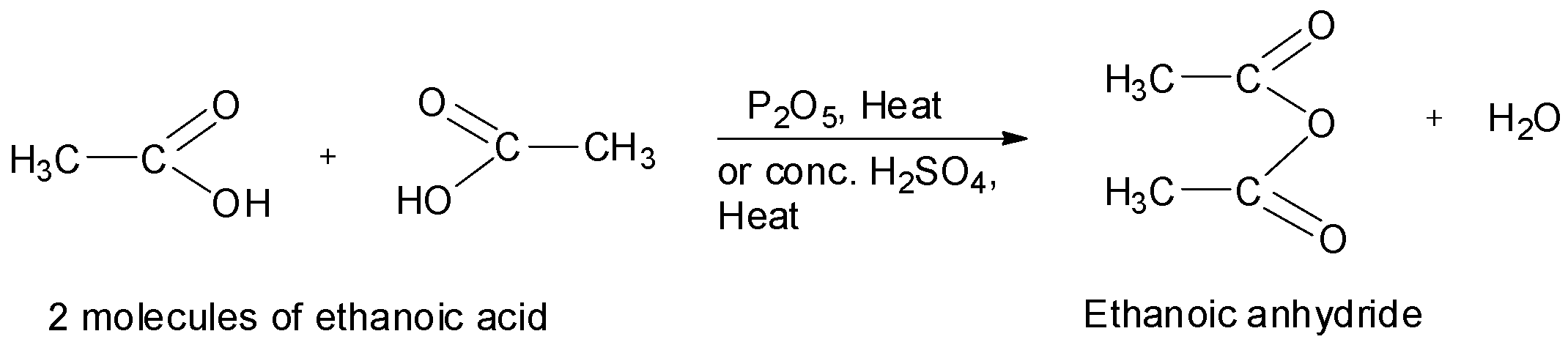
Similarly, propanoic anhydride can be prepared from propanoic acid. Now, the structure of propanoic acid is as shown below.
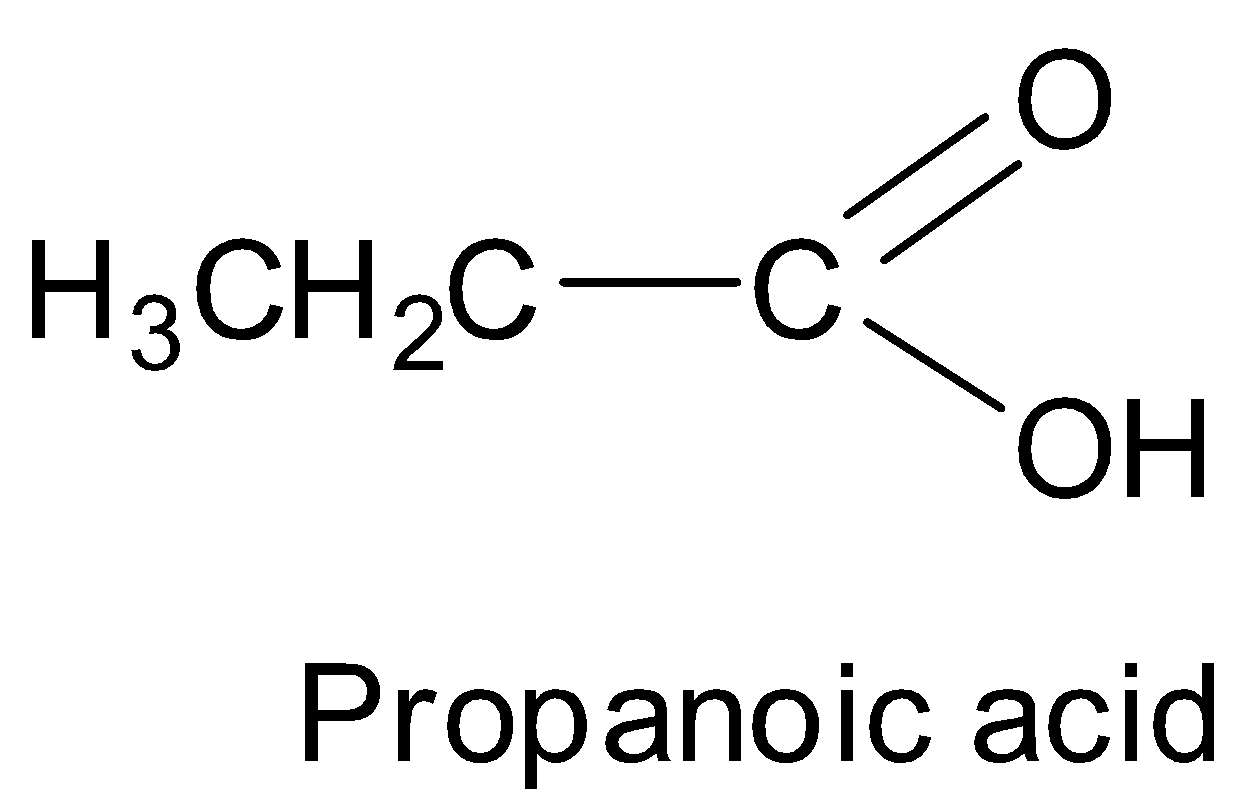
We can see that propanoic acid has 3 carbon atoms.
Thus, propanoic anhydride will be obtained when two molecules of propanoic acid is heated with phosphorus pentoxide or concentrated sulphuric acid.
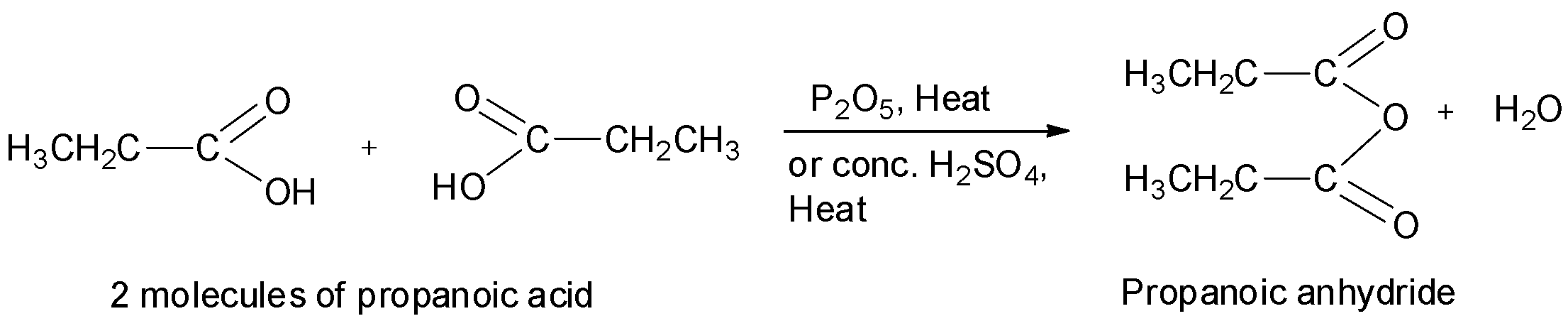
Thus, we can see that propanoic acid has the structure:
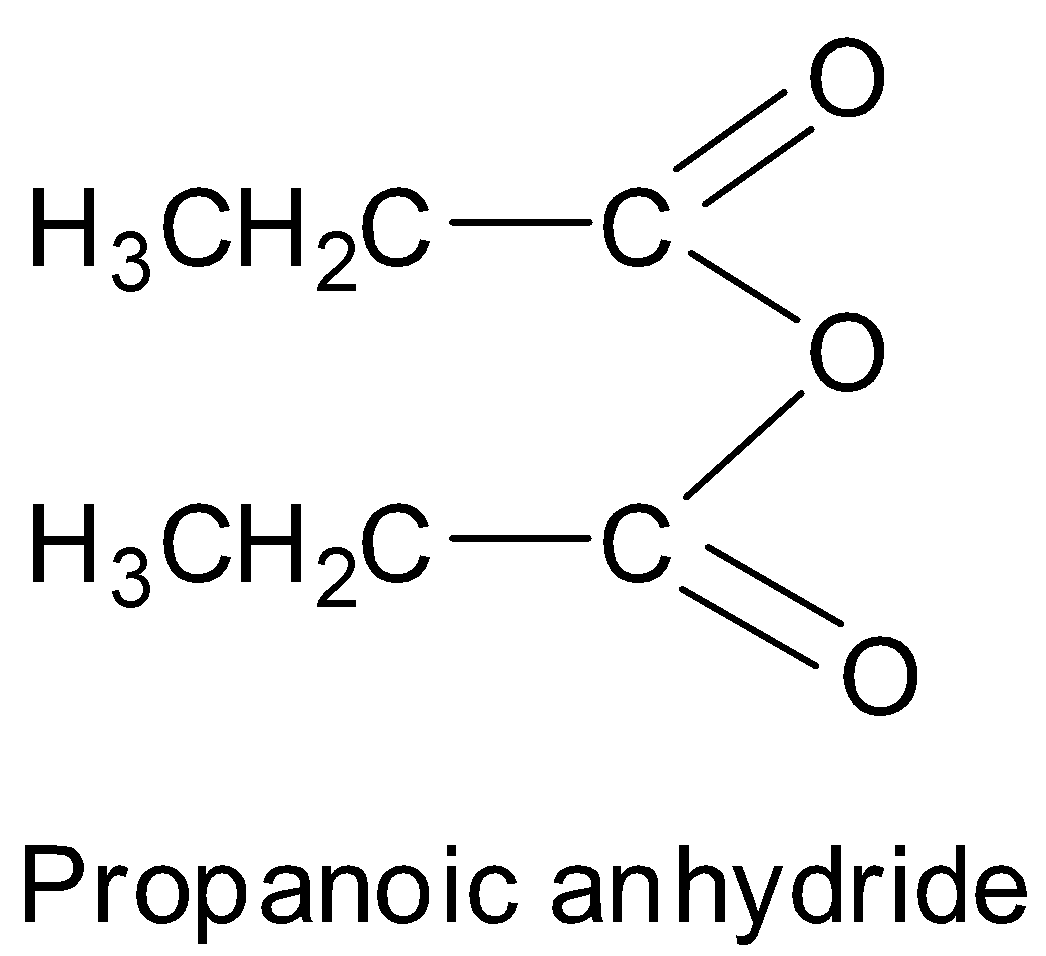
This can also be written in the form of formula as $ {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOCOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}} $ or $ {\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CO}}} \right)_{\text{2}}}{\text{O}} $ . Hence, option A is correct.
Option B is incorrect as $ {\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}}} \right)_{\text{2}}}{\text{O}} $ is ethanoic anhydride. Option C is incorrect as $ {\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CO}}} \right)_{\text{2}}}{\text{O}} $ is butanoic anhydride. Option D is incorrect as $ {\text{C}}{{\text{H}}_{\text{3}}}{\text{COOCOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}} $ is ethanoic propanoic anhydride.
Note:
Acetic anhydrides can also be obtained by treating acid chlorides with carboxylic acids in the presence of pyridine as a base. The reaction is shown below.
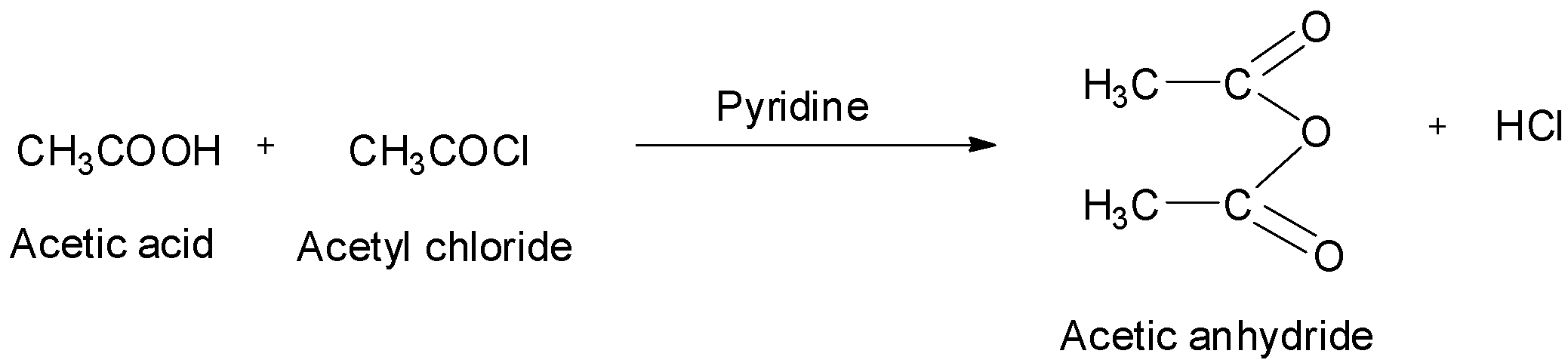
They can also be obtained by treating acid chlorides with sodium salts of carboxylic acids. The reaction is:
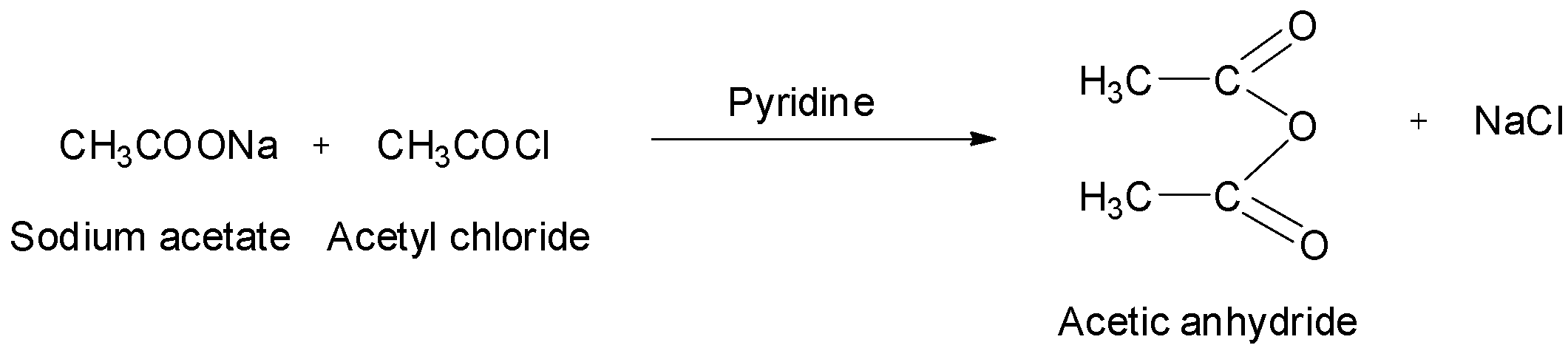
Complete Step By Step Answer:
When carboxylic acids are heated in the presence of a strong dehydrating agent such as phosphorus pentoxide or concentrated sulphuric acid, acid anhydrides are formed by the elimination of a molecule of water from two molecules of the acid. For example, ethanoic anhydride is obtained by the treatment of two molecules of ethanoic acid in presence of phosphorus pentoxide or concentrated sulphuric acid.

Similarly, propanoic anhydride can be prepared from propanoic acid. Now, the structure of propanoic acid is as shown below.

We can see that propanoic acid has 3 carbon atoms.
Thus, propanoic anhydride will be obtained when two molecules of propanoic acid is heated with phosphorus pentoxide or concentrated sulphuric acid.
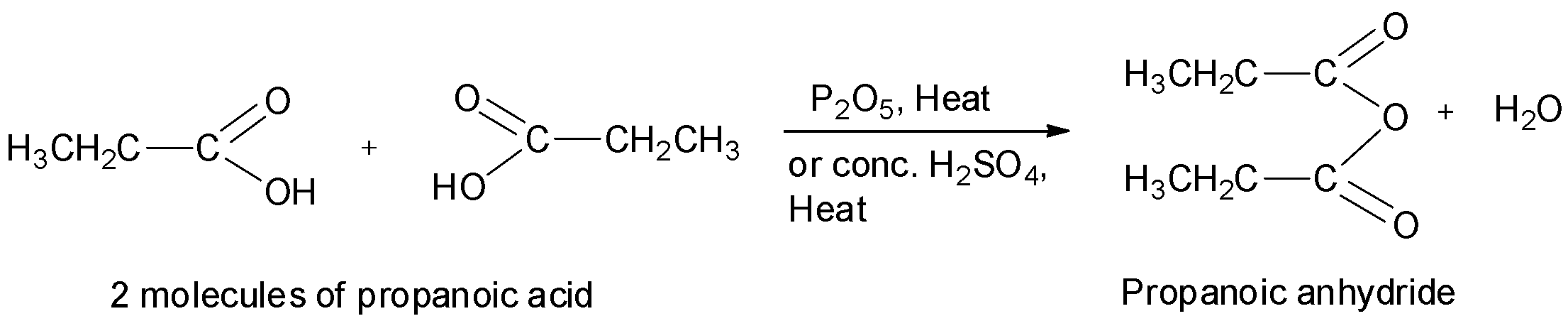
Thus, we can see that propanoic acid has the structure:

This can also be written in the form of formula as $ {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOCOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}} $ or $ {\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CO}}} \right)_{\text{2}}}{\text{O}} $ . Hence, option A is correct.
Option B is incorrect as $ {\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{CO}}} \right)_{\text{2}}}{\text{O}} $ is ethanoic anhydride. Option C is incorrect as $ {\left( {{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CO}}} \right)_{\text{2}}}{\text{O}} $ is butanoic anhydride. Option D is incorrect as $ {\text{C}}{{\text{H}}_{\text{3}}}{\text{COOCOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}} $ is ethanoic propanoic anhydride.
Note:
Acetic anhydrides can also be obtained by treating acid chlorides with carboxylic acids in the presence of pyridine as a base. The reaction is shown below.

They can also be obtained by treating acid chlorides with sodium salts of carboxylic acids. The reaction is:

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




