
If D- and L- glucose are enantiomers, are D- and L-fructose not enantiomers?
Answer
491.7k+ views
Hint: Enantiomers are a type of stereoisomers which are mirror images of each other and are non-superimposable on each other. Meaning if we stack up these mirror image molecules one over the other, the stack formed won’t be uniform in a uniform way.
Complete answer:
Enantiomers are the stereoisomers that have mirror images which are non-superimposable on each other. For example, consider D-Glucose and L-Glucose. For differentiating D and L configuration we use the Fischer Projection formula for carbohydrates. For D carbohydrates the -OH group on the penultimate (second last) carbon lies on the right-hand side and for L carbohydrates the -OH group lies on the left-hand side on the penultimate carbon atom. In Haworth projection for hexoses, in the cyclohexane ring the terminal carbon has the $ - C{H_2}OH$ pointing upward.
Consider the two compounds, D Glucose and L-Glucose. The structures can be given as:
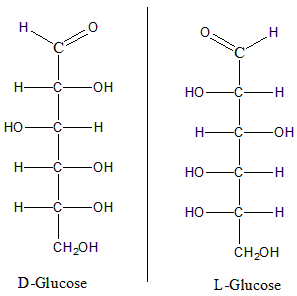
In here we can see that these both structures are mirror images of each other and both the images when stacked up over each other do not superimpose. Hence they are enantiomers. Now, consider the structures for D and L fructose.
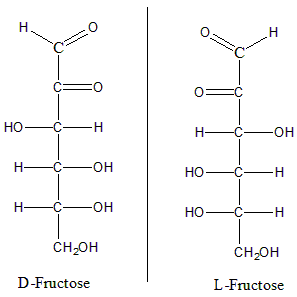
We can see that for D Fructose the -OH is on the Right-hand side and for L Fructose the -OH is on the left-hand side. We can see that both the structures are mirror images of each other and when places over one another cannot be superimposed. Hence these are also enantiomers.
Note:
Do not confuse D, L and d,l. The capital letters give us the information about the place of the -OH on the structure. It is nowhere related to the direction in which the plane polarized light is rotated. d and l give us the information about the direction of the light.
Complete answer:
Enantiomers are the stereoisomers that have mirror images which are non-superimposable on each other. For example, consider D-Glucose and L-Glucose. For differentiating D and L configuration we use the Fischer Projection formula for carbohydrates. For D carbohydrates the -OH group on the penultimate (second last) carbon lies on the right-hand side and for L carbohydrates the -OH group lies on the left-hand side on the penultimate carbon atom. In Haworth projection for hexoses, in the cyclohexane ring the terminal carbon has the $ - C{H_2}OH$ pointing upward.
Consider the two compounds, D Glucose and L-Glucose. The structures can be given as:

In here we can see that these both structures are mirror images of each other and both the images when stacked up over each other do not superimpose. Hence they are enantiomers. Now, consider the structures for D and L fructose.

We can see that for D Fructose the -OH is on the Right-hand side and for L Fructose the -OH is on the left-hand side. We can see that both the structures are mirror images of each other and when places over one another cannot be superimposed. Hence these are also enantiomers.
Note:
Do not confuse D, L and d,l. The capital letters give us the information about the place of the -OH on the structure. It is nowhere related to the direction in which the plane polarized light is rotated. d and l give us the information about the direction of the light.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




