
If ${{\text{K}}_{\text{a}}}$ more or $\text{p}{{\text{K}}_{\text{a}}}$ is less, then more stronger is the acid.
Ionic order: $\text{HCOOH}>{{\text{C}}_{6}}{{\text{H}}_{5}}\text{COOH}$
Answer
590.4k+ views
Hint: The acidic strength is decided by its ability to produce hydrogen ions or $\left[ {{\text{H}}^{+}} \right]$ ions. The production of $\left[ {{\text{H}}^{+}} \right]$ ions is determined by resonance and inductive effect in their respective structures. Let us study the structure of both the acids along with their strengths.
Complete step by step answer:
(1) Formic acid or $\text{HCOOH}$- This acid has one carbon atom and is the simplest carboxylic acid. Formic acid is also known as formaldehyde. Its structure is
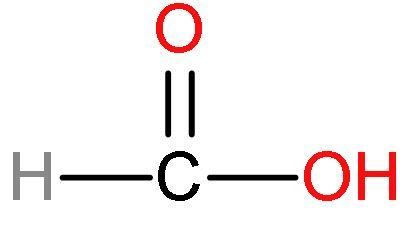
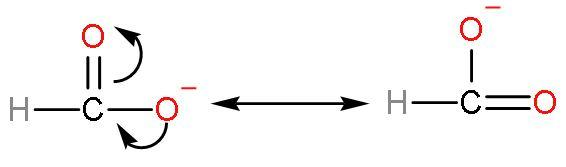
Formic acid has good acidic strength because there is such no presence of methyl groups in this acid. No methyl groups so, no inductive effect. When species have the tendency to donate electrons such as methyl or alkyl groups to a carbon chain, this charge is relayed through the chain and the effect is called the positive Inductive Effect. After losing $\left[ {{\text{H}}^{+}} \right]$ ions, it is highly stable due to highly stabilised resonating structures.
(2) Benzoic acid or ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{COOH}$- Benzoic acid is an aromatic carboxylic acid. Benzoic acid has a carboxyl group attached to the benzene ring. There is resonance with benzene and the $-\text{COOH}$ group. This decreases the acidity of this acid.
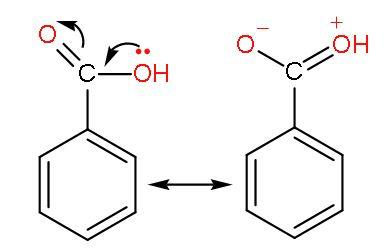
The phenyl group shows –I effect which attracts the electrons thus, helping in increasing the polarity of $-\text{COOH}$ bond for easy ionization of $\left[ {{\text{H}}^{+}} \right]$ ions. The resonance of $\text{C=O}$ with benzene ring occurs as
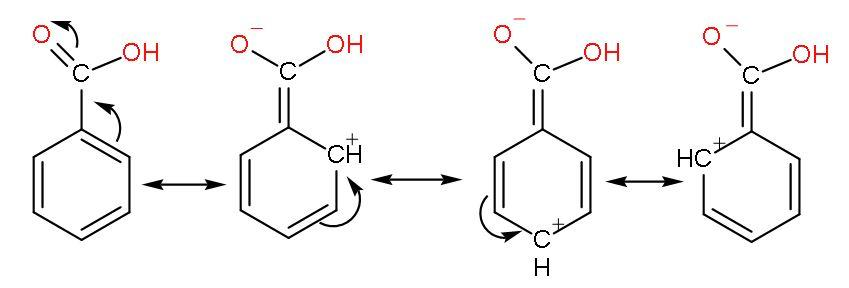
But its resonance with benzene rings forms unstable carbocations due to incomplete octet of carbon atoms (have only 6 electrons) makes benzoic acid less acidic than formic acid.
It is clear that formic acid is more acidic than benzoic acid, so acidic order $\text{HCOOH}>{{\text{C}}_{6}}{{\text{H}}_{5}}\text{COOH}$.
Note: It is generally thought that aliphatic carboxylic acids are stronger acids than aromatic carboxylic acids also involved in resonance with the benzene ring. The ${{\text{K}}_{\text{a}}}$ of formic acid and benzoic acid are $1.77\times {{10}^{-4}}$ and $6.3\times {{10}^{-5}}$. The difference is quite noticeable and distinguishable. So, it is clear that aromaticity plays a big role in acidity of the acids.
Complete step by step answer:
(1) Formic acid or $\text{HCOOH}$- This acid has one carbon atom and is the simplest carboxylic acid. Formic acid is also known as formaldehyde. Its structure is

Formic acid has good acidic strength because there is such no presence of methyl groups in this acid. No methyl groups so, no inductive effect. When species have the tendency to donate electrons such as methyl or alkyl groups to a carbon chain, this charge is relayed through the chain and the effect is called the positive Inductive Effect. After losing $\left[ {{\text{H}}^{+}} \right]$ ions, it is highly stable due to highly stabilised resonating structures.

(2) Benzoic acid or ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{COOH}$- Benzoic acid is an aromatic carboxylic acid. Benzoic acid has a carboxyl group attached to the benzene ring. There is resonance with benzene and the $-\text{COOH}$ group. This decreases the acidity of this acid.

The phenyl group shows –I effect which attracts the electrons thus, helping in increasing the polarity of $-\text{COOH}$ bond for easy ionization of $\left[ {{\text{H}}^{+}} \right]$ ions. The resonance of $\text{C=O}$ with benzene ring occurs as

But its resonance with benzene rings forms unstable carbocations due to incomplete octet of carbon atoms (have only 6 electrons) makes benzoic acid less acidic than formic acid.
It is clear that formic acid is more acidic than benzoic acid, so acidic order $\text{HCOOH}>{{\text{C}}_{6}}{{\text{H}}_{5}}\text{COOH}$.
Note: It is generally thought that aliphatic carboxylic acids are stronger acids than aromatic carboxylic acids also involved in resonance with the benzene ring. The ${{\text{K}}_{\text{a}}}$ of formic acid and benzoic acid are $1.77\times {{10}^{-4}}$ and $6.3\times {{10}^{-5}}$. The difference is quite noticeable and distinguishable. So, it is clear that aromaticity plays a big role in acidity of the acids.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




