
If the given aldohexose exists in its D-configuration, then the total number of stereoisomers in its pyranose form is:
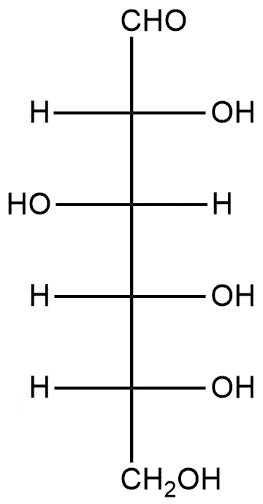
A. 4
B. 8
C. 16
D. 32

Answer
578.1k+ views
Hint: Stereoisomers are those compounds which have the same molecular formula but different arrangement of atoms in three dimensional space.
Complete Step by step solution: In stereochemistry, stereoisomerism or spatial isomerism is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of their atoms in space. This is similar to structural isomers which share the same molecular formula but the bond connections or their order differs. D and L glucose explains two different forms of glucose in which D-glucose corresponds to dextro and L corresponds to leavo. These are generally two forms of enantiomers as they are mirror images of each other. Pyranose is a type of saccharides that have a chemical structure which includes a six-membered ring consisting of five carbon atoms and one oxygen atom. There may be other carbons external to the ring. The name derives from its similarity to the oxygen heterocyclic pyran but the pyranose ring does not have double bonds. Pyranose contains 5 chiral carbon atoms and chiral centers are 3. Thus we know the rule of calculating stereoisomers is:
Number of stereoisomers = \[{{2}^{n}}\]
Where n is number of chiral centers, in this
Number of stereoisomers = \[{{2}^{3}}\]
= 8
Thus, the total number of stereoisomers in the pyranose ring is 8, option B is correct.
Note: The pyranose ring is formed by the reaction of the hydroxyl group on carbon 5 (C-5) of a sugar with the aldehyde at carbon 1. This forms an intramolecular hemiacetal. If reaction is between the C-4 hydroxyl and the aldehyde than furanose is formed instead. The pyranose form is thermodynamically more stable than the furanose form.
Complete Step by step solution: In stereochemistry, stereoisomerism or spatial isomerism is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of their atoms in space. This is similar to structural isomers which share the same molecular formula but the bond connections or their order differs. D and L glucose explains two different forms of glucose in which D-glucose corresponds to dextro and L corresponds to leavo. These are generally two forms of enantiomers as they are mirror images of each other. Pyranose is a type of saccharides that have a chemical structure which includes a six-membered ring consisting of five carbon atoms and one oxygen atom. There may be other carbons external to the ring. The name derives from its similarity to the oxygen heterocyclic pyran but the pyranose ring does not have double bonds. Pyranose contains 5 chiral carbon atoms and chiral centers are 3. Thus we know the rule of calculating stereoisomers is:
Number of stereoisomers = \[{{2}^{n}}\]
Where n is number of chiral centers, in this
Number of stereoisomers = \[{{2}^{3}}\]
= 8
Thus, the total number of stereoisomers in the pyranose ring is 8, option B is correct.
Note: The pyranose ring is formed by the reaction of the hydroxyl group on carbon 5 (C-5) of a sugar with the aldehyde at carbon 1. This forms an intramolecular hemiacetal. If reaction is between the C-4 hydroxyl and the aldehyde than furanose is formed instead. The pyranose form is thermodynamically more stable than the furanose form.
Recently Updated Pages
Master Class 12 Business Studies: Engaging Questions & Answers for Success

Master Class 12 Chemistry: Engaging Questions & Answers for Success

Master Class 12 Biology: Engaging Questions & Answers for Success

Class 12 Question and Answer - Your Ultimate Solutions Guide

Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE




