
In a conductometric titration experiment, a solution of $0.1MBa{\left( {OH} \right)_2}$ is titrated against a solution of $0.1MMg\left( {S{O_4}} \right)$ and the conductance of the mixture is continuously measured. The correct variation of conductance of the reaction mixture with the titration volume of $MgS{O_4}$ is best represented by.
A)
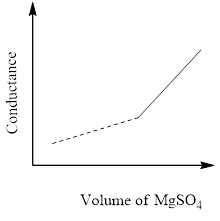
B)
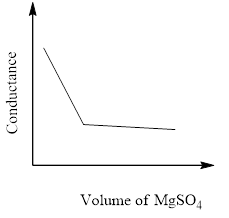
C)
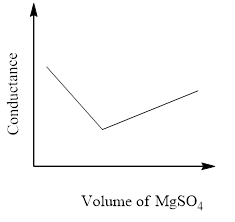
D)
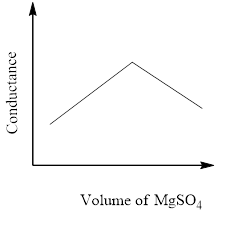 .
.




Answer
579.3k+ views
Hint: We know that the conductometric titration may be a laboratory method of quantitative chemical analysis wont to identify the concentration of a given analyte during a mixture. Conductometric titration involves the frequent adding of a reactant to a reaction mixture and consequently the records of the resultant change within the electrolytic conductivity of the reaction mixture
Complete step by step answer:
First we see the principle of the conductometric titration.
The principle of the conductometric titration is in a titration process, one ion is replaced with another and therefore the variation within the ionic conductivities of those ions directly impacts on the common electrolytic conductivity of the solution.
The conductivity that's measured in an electrolyte solution depends on the sort and concentration of the ions. As long since the reaction is taking its course the conductivity drops when the quality solution is in excess, the conductivity rises again.
So, the correct answer is Option C.
Note:
Now we can discuss about the advantages and drawbacks of conductometric titration as,
Advantages Conductometric Titration:
This process is extremely useful within the titrations of very dilute solutions and weak acids.
The end-point of this method of titration is extremely sharp and accurate in comparison to a couple of other titration processes.
This type of titration is applicable for solutions that are colored or turbid, and that the endpoint of the titration with normal indicators can't be observed easily by the human eye.
Conductometric titration has numerous applications in acid-base titrations, redox titrations, precipitation titrations, and sophisticated titrations.
The major drawbacks of this sort of titration include:
Only a couple of specific redox titrations are often through with the assistance of this process. This is often because the conductivity of the answer is masked by relatively high hydronium ion concentration.
Complete step by step answer:
First we see the principle of the conductometric titration.
The principle of the conductometric titration is in a titration process, one ion is replaced with another and therefore the variation within the ionic conductivities of those ions directly impacts on the common electrolytic conductivity of the solution.
The conductivity that's measured in an electrolyte solution depends on the sort and concentration of the ions. As long since the reaction is taking its course the conductivity drops when the quality solution is in excess, the conductivity rises again.
So, the correct answer is Option C.
Note:
Now we can discuss about the advantages and drawbacks of conductometric titration as,
Advantages Conductometric Titration:
This process is extremely useful within the titrations of very dilute solutions and weak acids.
The end-point of this method of titration is extremely sharp and accurate in comparison to a couple of other titration processes.
This type of titration is applicable for solutions that are colored or turbid, and that the endpoint of the titration with normal indicators can't be observed easily by the human eye.
Conductometric titration has numerous applications in acid-base titrations, redox titrations, precipitation titrations, and sophisticated titrations.
The major drawbacks of this sort of titration include:
Only a couple of specific redox titrations are often through with the assistance of this process. This is often because the conductivity of the answer is masked by relatively high hydronium ion concentration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




