
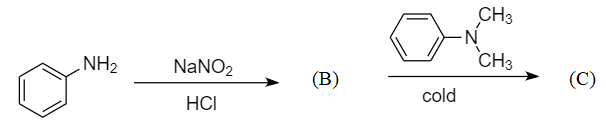
In a reaction of aniline, a coloured product C was obtained:
The structure of C would be:
(A)
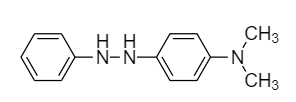
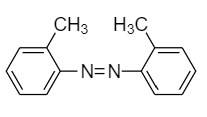
(B)
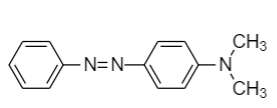
(C)
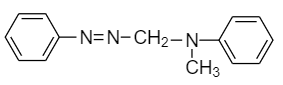
(D)




Answer
518.4k+ views
Hint: We know that azo-coupling reactions: These reactions are types of electrophilic substitution reactions in which diazonium salt plays a role of an electrophile and attacks the para position of the activated aromatic compounds and formation of azo-dye takes place.
Complete answer:
In the given reaction sequence, aniline is taken as the reactant which is an activated aromatic compound because of the presence of $ N{H_2} $ group which stabilizes the cationic intermediates formed between the substitution reaction by showing the $ + I $ effect.
So, the reaction mechanism followed is given below:
Step-1: When the primary aromatic amines like aniline react with nitrous acid in the presence of dilute hydrochloric acid, then diazonium salt is formed. This reaction is known as diazotization reaction. The reaction is as follows:
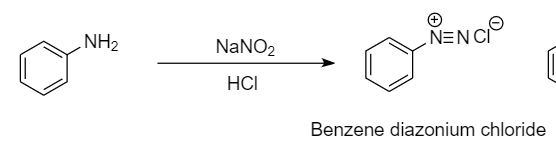
Hence product B formed is benzene diazonium chloride.
Step-2: When a diazonium compound reacts with another aromatic compound in cold conditions, then a coupling reaction takes place along with the removal of hydrochloric acid. The compound formed after the reaction is known as azo dye compound. The reaction is as follows:
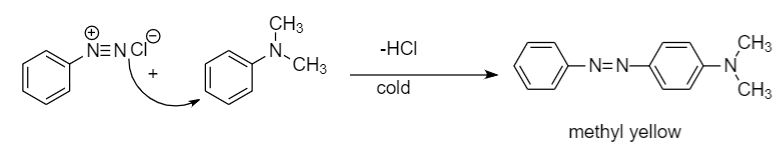
Hence product C formed is methyl yellow.
Therefore, option (B) is the correct answer.
Note:
Remember that in coupling reactions, the electrophilic attack of the diazonium compound is always preferred at the para position of the aromatic compound. But in case if the para position is already occupied by a functional group, then attack will take place at the ortho position of the aromatic compound.
Complete answer:
In the given reaction sequence, aniline is taken as the reactant which is an activated aromatic compound because of the presence of $ N{H_2} $ group which stabilizes the cationic intermediates formed between the substitution reaction by showing the $ + I $ effect.
So, the reaction mechanism followed is given below:
Step-1: When the primary aromatic amines like aniline react with nitrous acid in the presence of dilute hydrochloric acid, then diazonium salt is formed. This reaction is known as diazotization reaction. The reaction is as follows:

Hence product B formed is benzene diazonium chloride.
Step-2: When a diazonium compound reacts with another aromatic compound in cold conditions, then a coupling reaction takes place along with the removal of hydrochloric acid. The compound formed after the reaction is known as azo dye compound. The reaction is as follows:

Hence product C formed is methyl yellow.
Therefore, option (B) is the correct answer.
Note:
Remember that in coupling reactions, the electrophilic attack of the diazonium compound is always preferred at the para position of the aromatic compound. But in case if the para position is already occupied by a functional group, then attack will take place at the ortho position of the aromatic compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




