
In a set of reactions p - nitrotoluene yielded a product E. The product E would be:
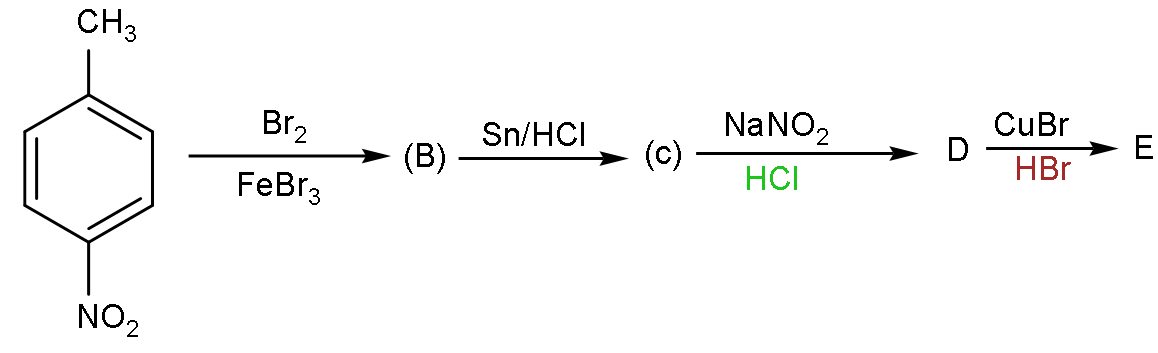
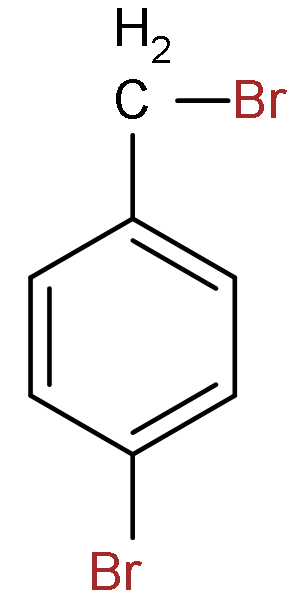
A.
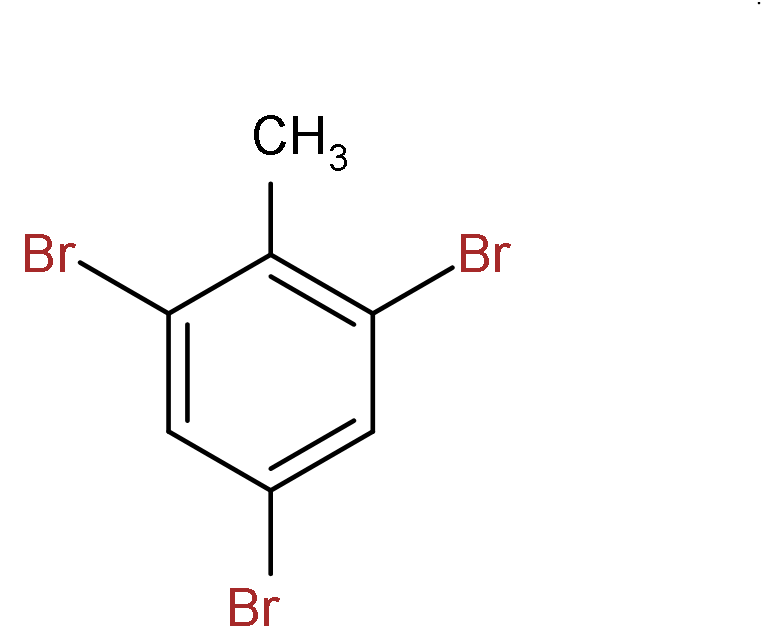
B.
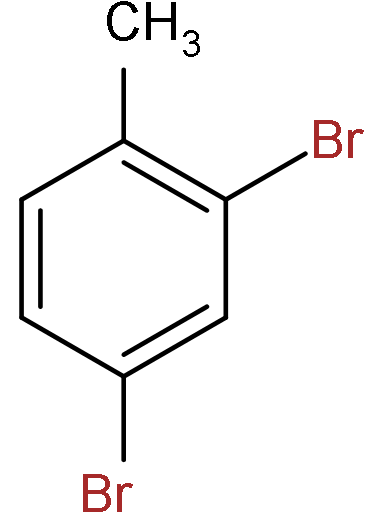
C.
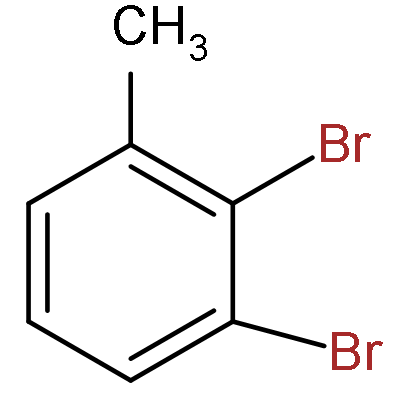
D.





Answer
579.3k+ views
Hint: For this problem, we have to write the complete reaction step by step in which firstly the bromination will take place due to which the bromine will attach on ortho position. Further by reducing the nitro group and then by forming diazonium ion, it will replace it with bromine.
Complete step by step answer:
- In the given question, we have to explain the product E which will be formed when the first reactant will react with the given agents.
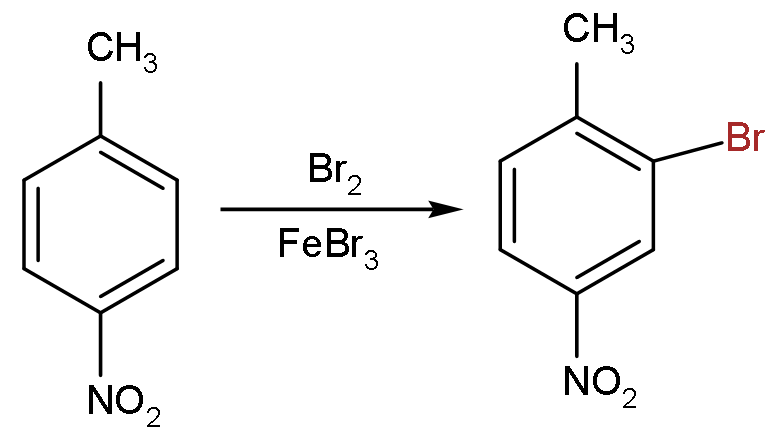
- Now, in the first step, we can see that when bromine and iron bromide react with 4-nitromethylbenzene they substitute the hydrogen atoms from the ortho position to the methyl group with bromine.
- So, the product formed will be:
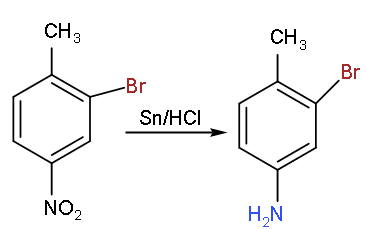
- Now, when this mono brominated product will react with tin in the presence of hydrochloric acid then it will reduce the nitro group to the amine group.
- As we know that in the reduction process the addition of the hydrogen atom takes place by replacing the oxygen atom so we will get:
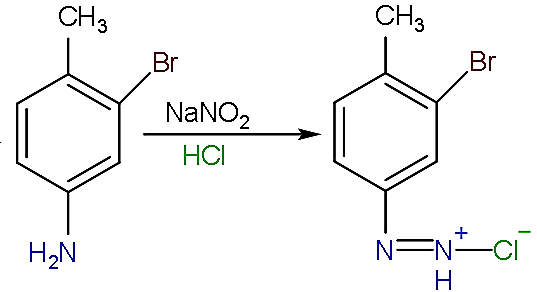
- Now, this the compound (c) formed reacts with the sodium nitrate in the presence of hydrochloric acid to convert amine group into diazonium salt as shown:
- Now, in the last step, the Gattermann reaction will take place, in which the formation of the aromatic ring takes place to produce halide benzene such as bromobenzene, chlorobenzene, etc.
- So, the product formed will be:
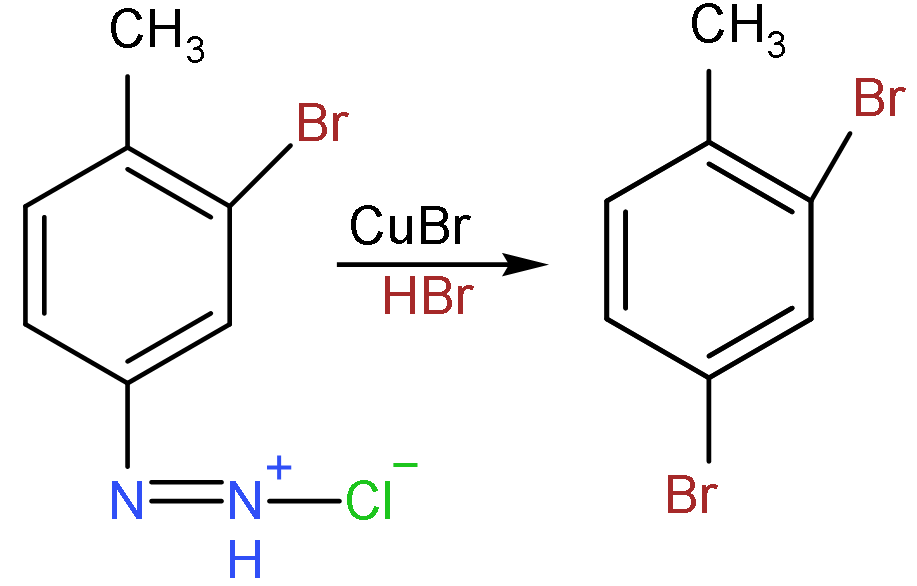
So, the correct answer is “Option C”.
Note: Gattermann reaction has another name that is Gattermann formylation reaction which was named after the scientist who discovered it i.e. Ludwig Gattermann. It shows similarity with the Friedal craft reaction which is used to attach the substituent to the aromatic ring.
Complete step by step answer:
- In the given question, we have to explain the product E which will be formed when the first reactant will react with the given agents.
- Now, in the first step, we can see that when bromine and iron bromide react with 4-nitromethylbenzene they substitute the hydrogen atoms from the ortho position to the methyl group with bromine.
- So, the product formed will be:

- Now, when this mono brominated product will react with tin in the presence of hydrochloric acid then it will reduce the nitro group to the amine group.
- As we know that in the reduction process the addition of the hydrogen atom takes place by replacing the oxygen atom so we will get:

- Now, this the compound (c) formed reacts with the sodium nitrate in the presence of hydrochloric acid to convert amine group into diazonium salt as shown:

- Now, in the last step, the Gattermann reaction will take place, in which the formation of the aromatic ring takes place to produce halide benzene such as bromobenzene, chlorobenzene, etc.
- So, the product formed will be:

So, the correct answer is “Option C”.
Note: Gattermann reaction has another name that is Gattermann formylation reaction which was named after the scientist who discovered it i.e. Ludwig Gattermann. It shows similarity with the Friedal craft reaction which is used to attach the substituent to the aromatic ring.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




