
In a/an_____________ the valence band and conduction band overlap with each other.
A) Insulator
B) Semiconductor
C) Conductor
D) Super conductor
Answer
582.9k+ views
Hint: The state of the electrons that have different energy values within specific ranges. Remember that if there is some gap between the valence band and conduction band electrons require more energy for the transition to a higher energy state. And if the bands overlap with each other there will be more flow electrons and
Complete step by step answer:
The separation between the bottom of the conduction band and the top of the valence band is called the energy gap.
The band theory of solids is used to classify the materials into conductor, insulators, and semiconductor
Conductors: Conductors are those substances which allow the electric current through them easily. It is because; there are large numbers of free electrons available in a conductor. In conductor’s valence band and conduction band overlap with each other so that the energy gap${E_g} = 0$. because at room temperature itself electron in the outermost orbit comes out of the atom and it becomes a free electron. these free electrons are having more energy compare to bound electrons. Free electrons are responsible for the conduction process. Thus, we call it a conduction band consists of free electrons. Consequently, electrons are free to move, within the substance from the valence band to the conduction band. As a result, a very large number of electrons are available for conduction and such materials are called conductors. Therefore, even a small amount of potential difference is enough to develop an electric current. Thus, the energy gap is zero.
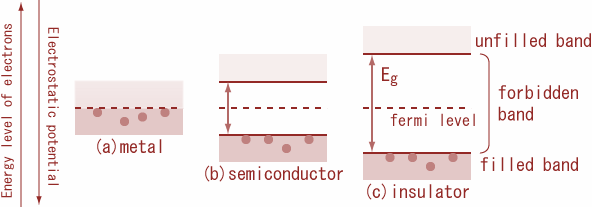
Additional information:
Insulator: Insulators are those substances which do not allow the electric current to pass through it. In insulators, the valence band is full, while the conduction band is empty. Therefore, a high electric field is required to move an electron from the valence band to the conduction band.
Semiconductors: Semiconductors are those substances whose electrical conductivity lies in between insulator and conductor. In a semiconductor, the valence band is almost filled and the conduction band is almost empty. The energy gap between the valence band and conduction is very small. At room temperature, some electrons from the valence band cross over to the conduction band, giving rise to little conductivity to the semiconductors. As temperature increases, more valence electrons cross over to the conduction band, and conductivity increases.
Hence the correct option is (C).
Note:
Conductors possess high electric and thermal conductivities. All metals are conductors.
Conductors are solids having low resistivity and high conductivity.
Complete step by step answer:
The separation between the bottom of the conduction band and the top of the valence band is called the energy gap.
The band theory of solids is used to classify the materials into conductor, insulators, and semiconductor
Conductors: Conductors are those substances which allow the electric current through them easily. It is because; there are large numbers of free electrons available in a conductor. In conductor’s valence band and conduction band overlap with each other so that the energy gap${E_g} = 0$. because at room temperature itself electron in the outermost orbit comes out of the atom and it becomes a free electron. these free electrons are having more energy compare to bound electrons. Free electrons are responsible for the conduction process. Thus, we call it a conduction band consists of free electrons. Consequently, electrons are free to move, within the substance from the valence band to the conduction band. As a result, a very large number of electrons are available for conduction and such materials are called conductors. Therefore, even a small amount of potential difference is enough to develop an electric current. Thus, the energy gap is zero.

Additional information:
Insulator: Insulators are those substances which do not allow the electric current to pass through it. In insulators, the valence band is full, while the conduction band is empty. Therefore, a high electric field is required to move an electron from the valence band to the conduction band.
Semiconductors: Semiconductors are those substances whose electrical conductivity lies in between insulator and conductor. In a semiconductor, the valence band is almost filled and the conduction band is almost empty. The energy gap between the valence band and conduction is very small. At room temperature, some electrons from the valence band cross over to the conduction band, giving rise to little conductivity to the semiconductors. As temperature increases, more valence electrons cross over to the conduction band, and conductivity increases.
Hence the correct option is (C).
Note:
Conductors possess high electric and thermal conductivities. All metals are conductors.
Conductors are solids having low resistivity and high conductivity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




