
In acidic medium, \[{H_2}{O_2}\] changes \[C{r_2}{O_7}^{2 - }\] to \[Cr{O_5}\] which has two (-O-O-) bonds. Oxidation state of Cr in \[Cr{O_5}\] is:
(A) +5
(B) +3
(C) +6
(D) -10
Answer
593.4k+ views
Hint: In peroxide compounds, oxidation number of oxygen atoms is taken as -1 . We can calculate the overall charge of a compound by just adding respective oxidation numbers of all atoms present in the compound. From this expression, we can find the oxidation number of Cr.
Complete answer:
Let’s see the structure of \[Cr{O_5}\] to get ahead.
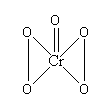
You need to remember that \[Cr{O_5}\] contains two -O-O- linkages. So, in short \[Cr{O_5}\] has four oxygen atoms that are in peroxide linkage.
Now that is a rule that if oxygen atom is involved in a peroxide linkage, then both the oxygen have oxidation number +1 which is different from the normal oxidation state of oxygen atom.
Let’s calculate the oxidation state of Cr in \[Cr{O_5}\].
Overall charge on compound= Oxidation state of Cr + 4(Oxidation state of oxygen in peroxide form) + Oxidation state of other oxygen atom
0 = Oxidation state of Cr + 4(-1) + (-2)
0 = Oxidation state of Cr - 4 - 2
Oxidation state of Cr = 4 + 2
Oxidation state of Cr = +6
Hence we can say that the correct answer of this question is (C) +6.
Additional Information:
- Remember that the normal oxidation state of an oxygen atom is -2 .
- In case that compound contains -O-O- (peroxide) linkage, then the numbers of oxygen involved in peroxide linkage have an oxidation number equal to -1 .
- When oxygen atom is present in superoxide from, those atoms have an oxidation number of -0.5 .
Note: Do not consider oxidation number of oxygen atoms in peroxide linkage equal to (-2) as this mistake often occurs. In case there is overall charge on the compound, do not forget to include it in the calculation of oxidation number.
Complete answer:
Let’s see the structure of \[Cr{O_5}\] to get ahead.

You need to remember that \[Cr{O_5}\] contains two -O-O- linkages. So, in short \[Cr{O_5}\] has four oxygen atoms that are in peroxide linkage.
Now that is a rule that if oxygen atom is involved in a peroxide linkage, then both the oxygen have oxidation number +1 which is different from the normal oxidation state of oxygen atom.
Let’s calculate the oxidation state of Cr in \[Cr{O_5}\].
Overall charge on compound= Oxidation state of Cr + 4(Oxidation state of oxygen in peroxide form) + Oxidation state of other oxygen atom
0 = Oxidation state of Cr + 4(-1) + (-2)
0 = Oxidation state of Cr - 4 - 2
Oxidation state of Cr = 4 + 2
Oxidation state of Cr = +6
Hence we can say that the correct answer of this question is (C) +6.
Additional Information:
- Remember that the normal oxidation state of an oxygen atom is -2 .
- In case that compound contains -O-O- (peroxide) linkage, then the numbers of oxygen involved in peroxide linkage have an oxidation number equal to -1 .
- When oxygen atom is present in superoxide from, those atoms have an oxidation number of -0.5 .
Note: Do not consider oxidation number of oxygen atoms in peroxide linkage equal to (-2) as this mistake often occurs. In case there is overall charge on the compound, do not forget to include it in the calculation of oxidation number.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




