
In alkyl halide X reacts with sodium to form 3,8-dimethyl decane. What is X?
(A)
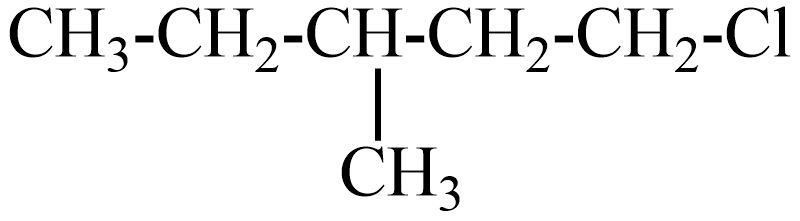
(B)
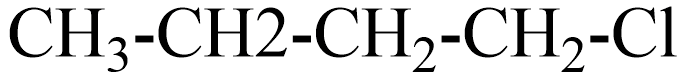
(C)
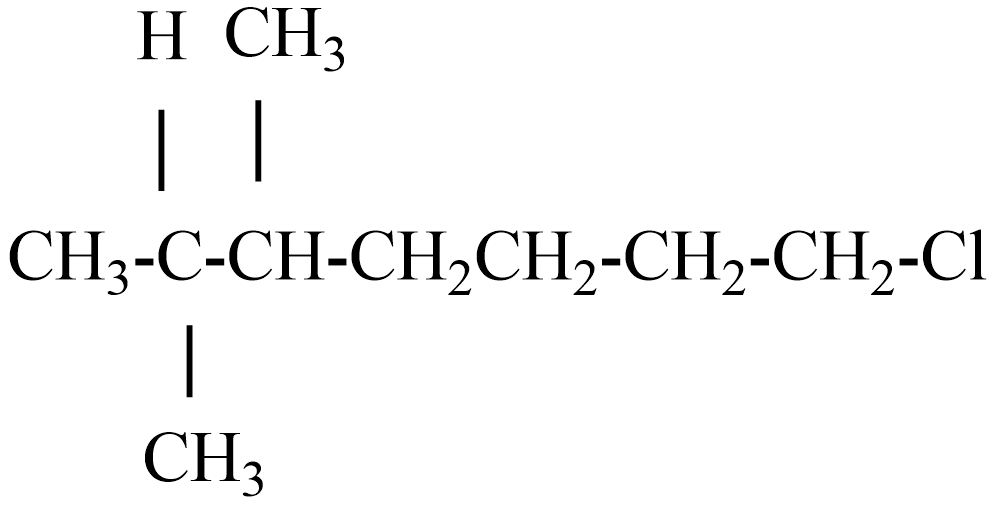
(D)
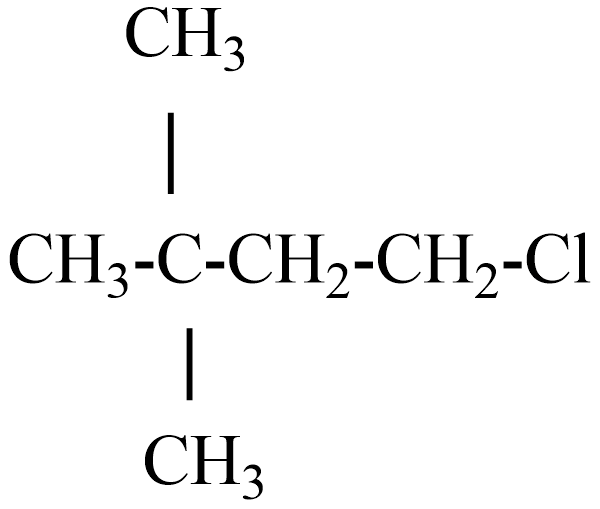

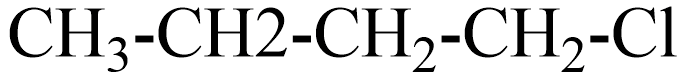
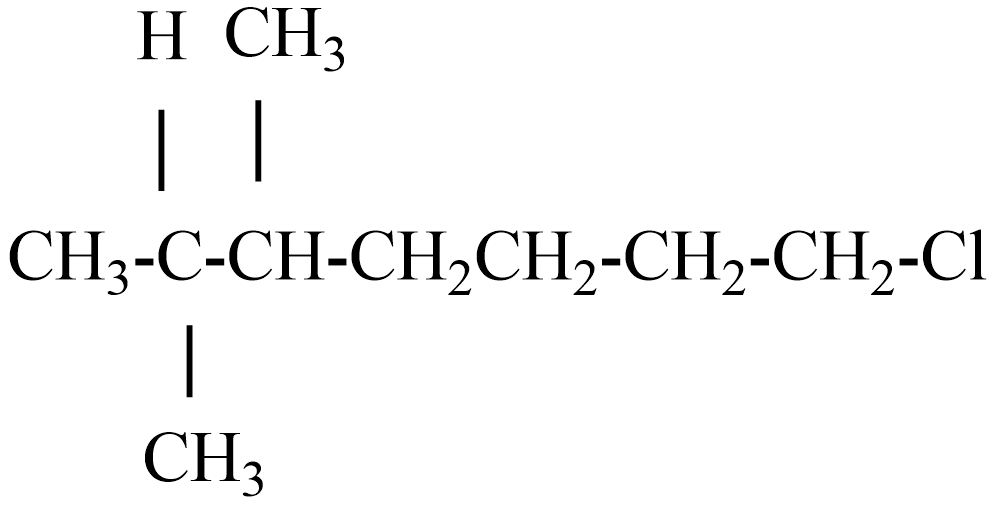
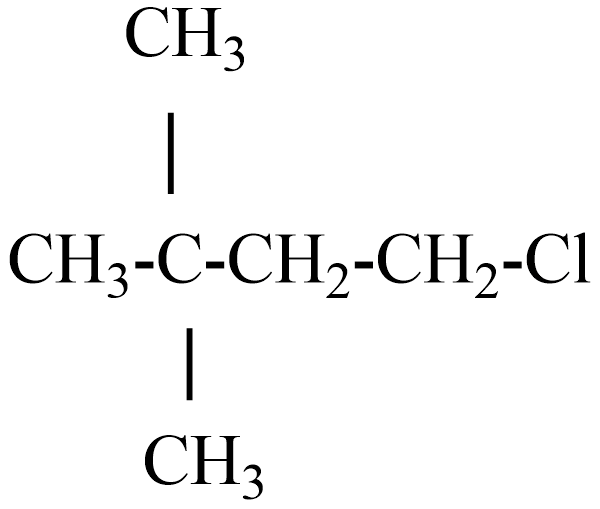
Answer
577.8k+ views
Hint: Alkyl halides on treatment with sodium metal in dry ethereal (free from moisture) solution give higher alkane. This reaction is known as the Wurtz reaction and is used for the preparation of higher alkanes containing an even number of carbon atoms.
Complete answer:
According to Wurtz reaction, two metal halides react in the presence of sodium metal and dry ether solution will form higher alkanes.
Applying this statement to all compounds given and let us see with alkyl halide will give the product 3,8-dimethyl decane.
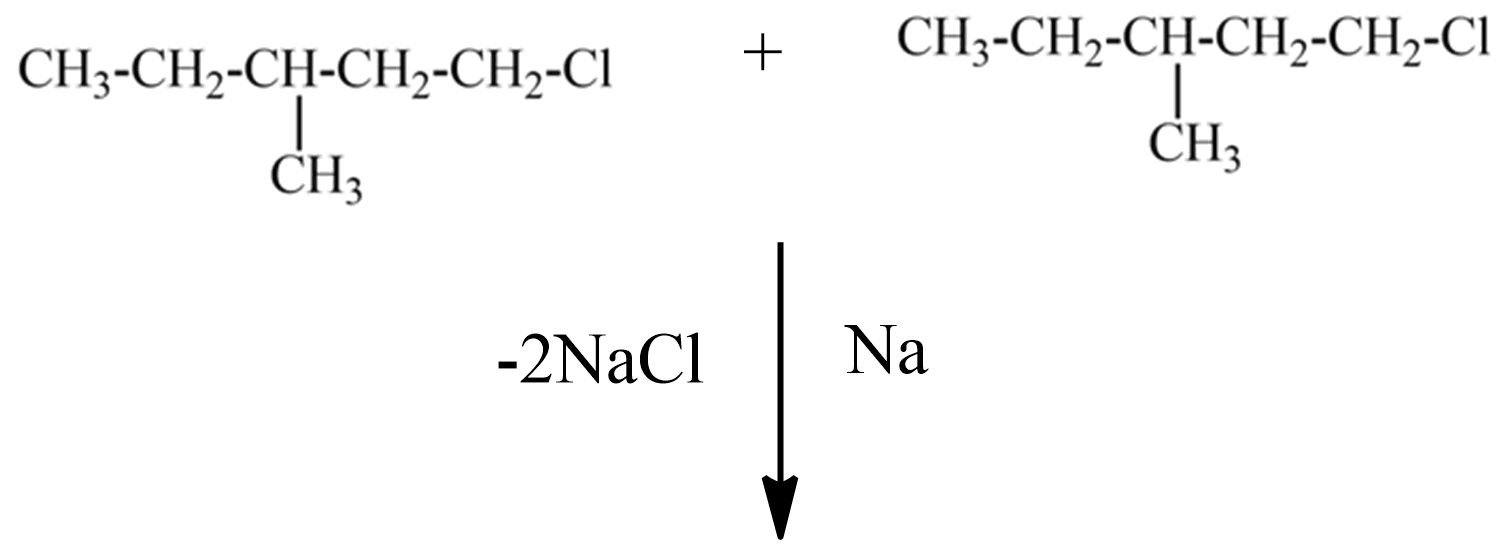
(A) the IUPAC name of this compound is chloro-3-methyl pentane.
If this compound obeys the Wurtz reaction rule, reacts with sodium metal in the presence of dry ether solution, the reaction follows,

(B) The IUPAC name of this compound is chlorobutane. If this compound obeys the Wurtz reaction rule, the product is n-octane.
(C) the IUPAC name of the given compound is chloro-2,3-dimethyl heptane.
If this compound obeys the Wurtz reaction rule, while react with Na metal the product is 2,3,8,9-tetramethyl tetradecane.
(D) The IUPAC name of this compound is chloro-2,2-dimethylbutane.
If this compound obeys the Wurtz reaction rule, while reacting with Na metal the product is 2, 2,5,5- tetramethyl octane.
From all four compounds, option A is (the correct answer) the alkyl halide X.
Note:
If two different alkyl halides are used in Wurtz reaction to prepare an alkane with an odd number of carbon atoms, a mixture of three alkanes is produced. The reason is that the two alkyl halides, in addition to reacting with each other also react among themselves giving a mixture of three alkanes.
Complete answer:
According to Wurtz reaction, two metal halides react in the presence of sodium metal and dry ether solution will form higher alkanes.
Applying this statement to all compounds given and let us see with alkyl halide will give the product 3,8-dimethyl decane.
(A) the IUPAC name of this compound is chloro-3-methyl pentane.
If this compound obeys the Wurtz reaction rule, reacts with sodium metal in the presence of dry ether solution, the reaction follows,


(B) The IUPAC name of this compound is chlorobutane. If this compound obeys the Wurtz reaction rule, the product is n-octane.
(C) the IUPAC name of the given compound is chloro-2,3-dimethyl heptane.
If this compound obeys the Wurtz reaction rule, while react with Na metal the product is 2,3,8,9-tetramethyl tetradecane.
(D) The IUPAC name of this compound is chloro-2,2-dimethylbutane.
If this compound obeys the Wurtz reaction rule, while reacting with Na metal the product is 2, 2,5,5- tetramethyl octane.
From all four compounds, option A is (the correct answer) the alkyl halide X.
Note:
If two different alkyl halides are used in Wurtz reaction to prepare an alkane with an odd number of carbon atoms, a mixture of three alkanes is produced. The reason is that the two alkyl halides, in addition to reacting with each other also react among themselves giving a mixture of three alkanes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




