
In an galvanic cell, the salt bridge:
A. does not participate chemically in the cell reaction
B. stops the diffusion of ions from one electrode to another
C. is necessary for the occurrence of the cell reaction
D. ensures mixing of the two electrolytic solution
Answer
579.6k+ views
Hint: A most remarkable feature of oxidation-reduction reactions is that they can be carried out with the reactants separated in a space and linked only by an electrical connection that is to say, chemical energy is converted to electrical energy .
Step by step answer: Consider figure representation of galvanic cells which involve reaction between metallic zinc and cupric ion. One can see that the cell consists of two beakers, one of which contains the solution of $C{u^{2 + }}$ and a copper rod, the other $Z{n^{2 + }}$ solution and a zinc rod. A connection is made between two solutions by means of '' salt bridge”. It is a tube containing a solution of electrolyte, generally $N{H_4}N{O_3},KCl$ .
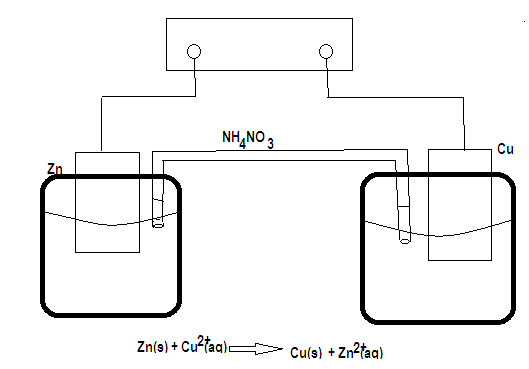
Flow of the solution from the salt bridge is prevented either by plugging the ends of the bridge by glass wool, or by using a salt dissolved in a gelatinous material as a bridge electrolyte. The purpose of the salt bridge is to prevent any net charge accumulation in either beaker, diffuse through the bridge and enter the left beaker at the same time there can be diffusion of positive ions from left to right if this diffusional change of ions do not occur, the net charge accumulating in the beaker would immediately stop the electron flow through the external surface and the oxidation reduction reaction would stop. Thus while the salt bridge does not participate chemically in the cell reaction, it is necessary for the cell to operate.
Hence from above we can conclude that the correct option is A.
Note: The galvanic cell mentioned above is represented in a short IUPAC cell notation as follows:
Zn/$ZnS{O_4}\parallel CuS{O_4}/Cu$ where $\parallel $ indicates the presence of salt bridge in the notation. As galvanic cell is an standard cell so students must be very familiar with the term.
Step by step answer: Consider figure representation of galvanic cells which involve reaction between metallic zinc and cupric ion. One can see that the cell consists of two beakers, one of which contains the solution of $C{u^{2 + }}$ and a copper rod, the other $Z{n^{2 + }}$ solution and a zinc rod. A connection is made between two solutions by means of '' salt bridge”. It is a tube containing a solution of electrolyte, generally $N{H_4}N{O_3},KCl$ .

Flow of the solution from the salt bridge is prevented either by plugging the ends of the bridge by glass wool, or by using a salt dissolved in a gelatinous material as a bridge electrolyte. The purpose of the salt bridge is to prevent any net charge accumulation in either beaker, diffuse through the bridge and enter the left beaker at the same time there can be diffusion of positive ions from left to right if this diffusional change of ions do not occur, the net charge accumulating in the beaker would immediately stop the electron flow through the external surface and the oxidation reduction reaction would stop. Thus while the salt bridge does not participate chemically in the cell reaction, it is necessary for the cell to operate.
Hence from above we can conclude that the correct option is A.
Note: The galvanic cell mentioned above is represented in a short IUPAC cell notation as follows:
Zn/$ZnS{O_4}\parallel CuS{O_4}/Cu$ where $\parallel $ indicates the presence of salt bridge in the notation. As galvanic cell is an standard cell so students must be very familiar with the term.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




