
In Down’s process, sodium is obtained by the electrolysis of:
A) Molten NaOH
B) Molten NaCl
C) Aqueous NaOH
D) Aqueous NaCl
Answer
547.8k+ views
Hint: The Downs' process is an electrochemical process. This process is carried out in the Down cell. The Downs cell was invented in 1922 by the American chemist James Cloyd Down.
Complete answer:
We know that electrolysis is a process by which electric current is passed through a substance to effect a chemical change. The chemical change is one by which a substance loses or gains electrons (oxidation or reduction).
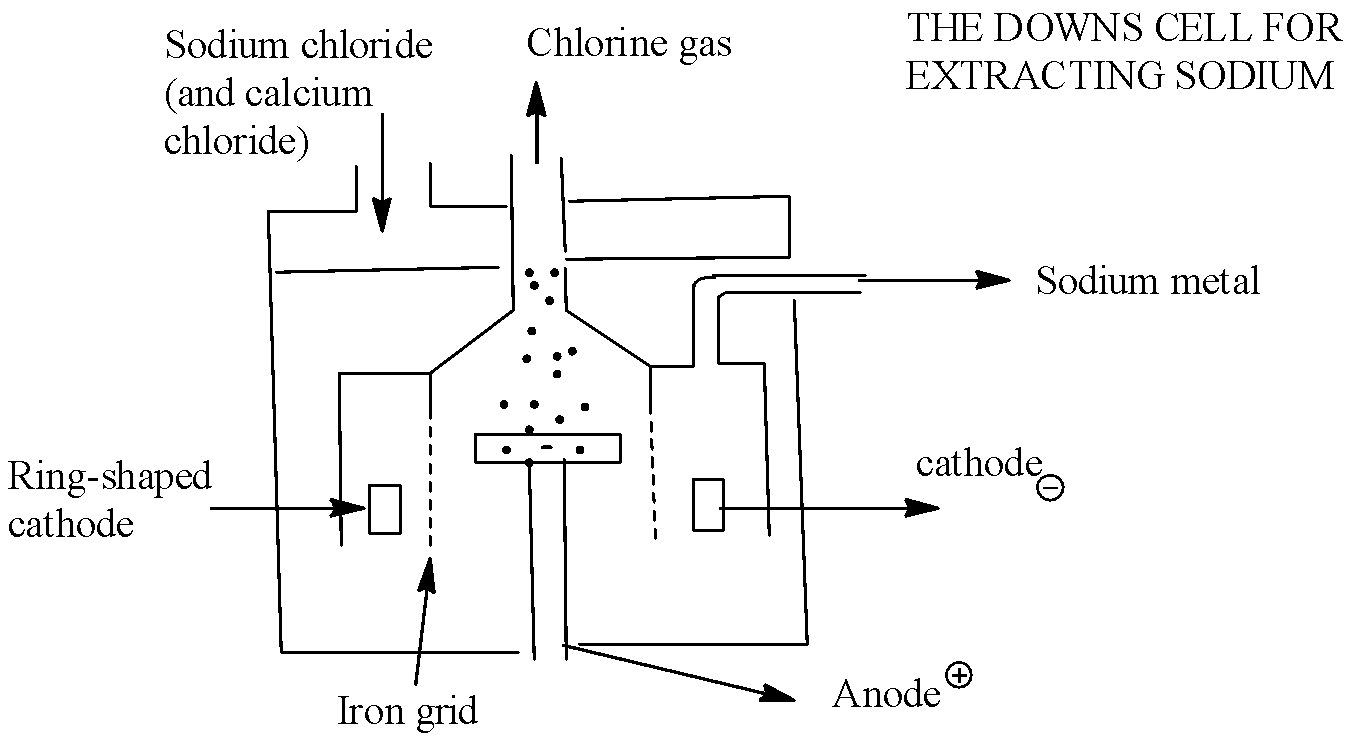
Down's process is used for extracting sodium by the electrolysis of molten sodium chloride. Chlorine is collected at the anode. Fused \[NaCl\]contains sodium and chloride ions. \[N{{a}^{+}}\]ions migrate to cathode where they are reduced to Na. \[C{{l}^{-}}\]ions migrate to anode and oxidised to form chlorine gas. When an electric current is passed through the molten mixture of NaCl, NaCl decomposes in to \[N{{a}^{+}}\]and \[C{{l}^{-}}\] ion. \[N{{a}^{+}}\]ions migrate towards the cathode while \[C{{l}^{-}}\] ions towards the anode.
At cathode:
\[2N{{a}^{+}}+2{{e}^{-}}\to 2Na(reduction)\]
At anode:
\[2C{{l}^{-}}\to C{{l}_{2}}+2{{e}^{-}}(oxidation)\]
Overall reaction:
\[\begin{align}
& 2N{{a}^{+}}+2{{e}^{-}}\to 2Na \\
& 2C{{l}^{-}}\to C{{l}_{2}}+2{{e}^{-}} \\
& \overline{2N{{a}^{+}}+2C{{l}^{-}}\to 2Na+C{{l}_{2}}} \\
\end{align}\]
So, from the above explanation we can say that the correct answer is option “B”.
Note:
This process is carried out in an electrolytic cell. KCl and KF are used to decrease the melting point of the mixture. The molten sodium collects in the cathode compartment and Chlorine is collected at the anode compartment.
Complete answer:
We know that electrolysis is a process by which electric current is passed through a substance to effect a chemical change. The chemical change is one by which a substance loses or gains electrons (oxidation or reduction).
Down's process is used for extracting sodium by the electrolysis of molten sodium chloride. Chlorine is collected at the anode. Fused \[NaCl\]contains sodium and chloride ions. \[N{{a}^{+}}\]ions migrate to cathode where they are reduced to Na. \[C{{l}^{-}}\]ions migrate to anode and oxidised to form chlorine gas. When an electric current is passed through the molten mixture of NaCl, NaCl decomposes in to \[N{{a}^{+}}\]and \[C{{l}^{-}}\] ion. \[N{{a}^{+}}\]ions migrate towards the cathode while \[C{{l}^{-}}\] ions towards the anode.
At cathode:
\[2N{{a}^{+}}+2{{e}^{-}}\to 2Na(reduction)\]
At anode:
\[2C{{l}^{-}}\to C{{l}_{2}}+2{{e}^{-}}(oxidation)\]
Overall reaction:
\[\begin{align}
& 2N{{a}^{+}}+2{{e}^{-}}\to 2Na \\
& 2C{{l}^{-}}\to C{{l}_{2}}+2{{e}^{-}} \\
& \overline{2N{{a}^{+}}+2C{{l}^{-}}\to 2Na+C{{l}_{2}}} \\
\end{align}\]

So, from the above explanation we can say that the correct answer is option “B”.
Note:
This process is carried out in an electrolytic cell. KCl and KF are used to decrease the melting point of the mixture. The molten sodium collects in the cathode compartment and Chlorine is collected at the anode compartment.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




