
In face centered cubic unit cell, edge length is:
a.) \[\dfrac{4}{\sqrt{3}}r\]
b.) \[\dfrac{4}{\sqrt{3}}r\]
c.) \[2r\]
d.) \[\dfrac{\sqrt{3}}{2}r\]
Answer
595.2k+ views
Hint: Question is talking about FCC from the solid state chapter, and here we have to find edge length in terms of radius of each atom for which we have to check the number of atoms on each edge or diagonal. And from a face centered cubic unit cell we can understand that it also has atoms on the face center.
Complete step by step solution:
Face-centered cubic lattice (fcc), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of each face of the unit cell. The simplest crystal structures are those in which there is only a single atom at each lattice point.
It has unit cell vectors a =b =c. The number of atoms in a unit cell is four (8×1/8 + 6×1/2 = 4). There are 26 metals that have the fcc lattice.
So, for finding edge length let’s assume length of edge is “a” and radius of atom is “r”.
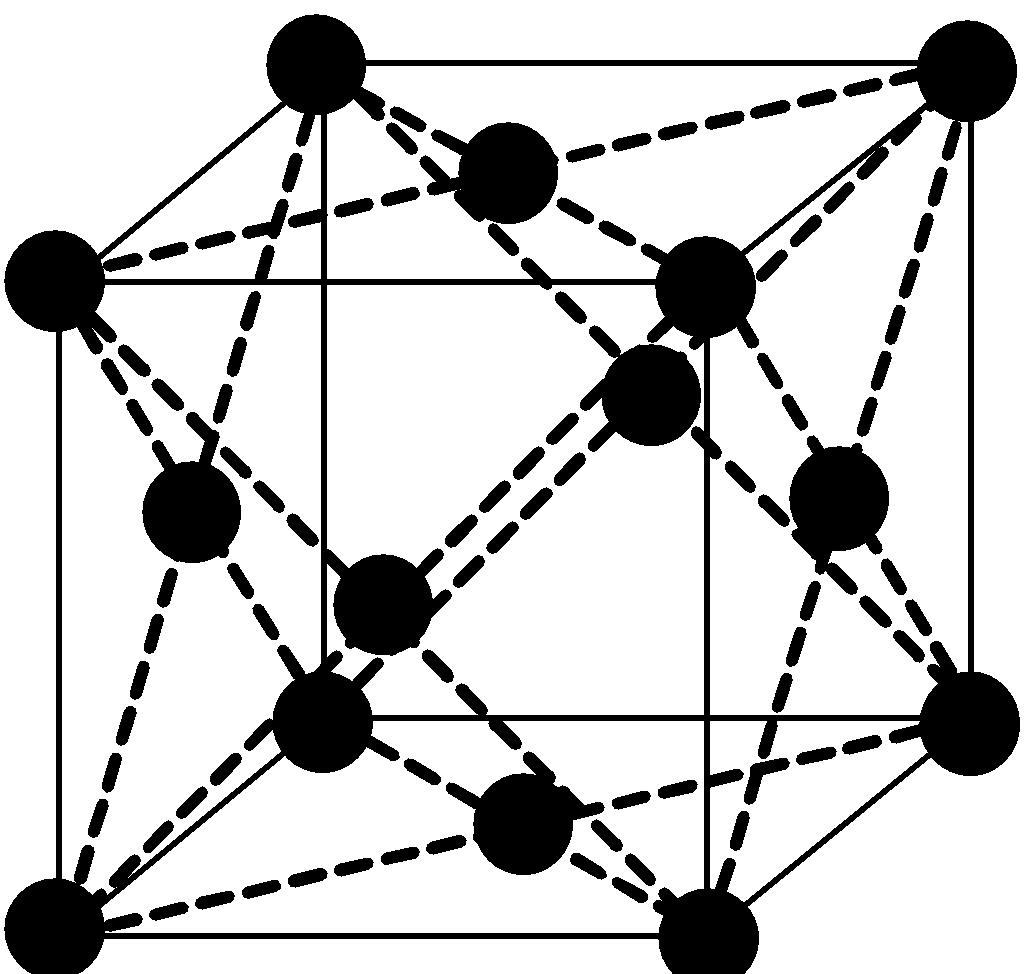
In FCC there is some gap between atoms which are on edge but there is no gap between the atoms on diagonal.
We will write the diagonal relation:
\[\sqrt{3}\] x (edge of unit cell) = 4 x radius of atom
\[\sqrt{3}\]a = 4 r
So, by rearranging:
‘a’ = \[\dfrac{4}{\sqrt{3}}\]r
Edge length of fcc unit cell = \[\dfrac{4}{\sqrt{3}}\]r
So, the correct answer is “Option A”.
Note: Here you should know that atoms on edge are not in contact with each other but atoms on diagonal are in touch with each other. In the fcc structures the spheres fill 74 % of the volume.
Complete step by step solution:
Face-centered cubic lattice (fcc), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of each face of the unit cell. The simplest crystal structures are those in which there is only a single atom at each lattice point.
It has unit cell vectors a =b =c. The number of atoms in a unit cell is four (8×1/8 + 6×1/2 = 4). There are 26 metals that have the fcc lattice.
So, for finding edge length let’s assume length of edge is “a” and radius of atom is “r”.


In FCC there is some gap between atoms which are on edge but there is no gap between the atoms on diagonal.
We will write the diagonal relation:
\[\sqrt{3}\] x (edge of unit cell) = 4 x radius of atom
\[\sqrt{3}\]a = 4 r
So, by rearranging:
‘a’ = \[\dfrac{4}{\sqrt{3}}\]r
Edge length of fcc unit cell = \[\dfrac{4}{\sqrt{3}}\]r
So, the correct answer is “Option A”.
Note: Here you should know that atoms on edge are not in contact with each other but atoms on diagonal are in touch with each other. In the fcc structures the spheres fill 74 % of the volume.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




