
In ${H_2}{O_2}$, the degree of hydrogen bonding is:
A) Same as in water
B) More than in water
C) Less than in water
D) Zero
Answer
573.6k+ views
Hint:Hydrogen bonding is a type of bond which formed between the two elements of two molecules. One can draw the structure of ${H_2}{O_2}$ and analyze how many hydrogen bonds can be present and compare them with the hydrogen bonds present in the water molecules.
Complete answer:
1) First of all we will learn about the concept of hydrogen bonding. Hydrogen bonding is the intermolecular type of bonding which is an intermolecular force.
2) The intermolecular force forms a special type of dipole-dipole attraction between a hydrogen atom bond and a strongly electronegative atom of another molecule present nearby which also possesses a lone pair of electrons.
3) Now let us analyze the structure ${H_2}{O_2}$ in which there are two oxygen molecules present. Each oxygen molecule will form two hydrogen bonds with two molecules of ${H_2}{O_2}$ which will result in the formation of four hydrogen bonds in a structure of hydrogen peroxide. The structure of hydrogen bonding ${H_2}{O_2}$ will be as below,
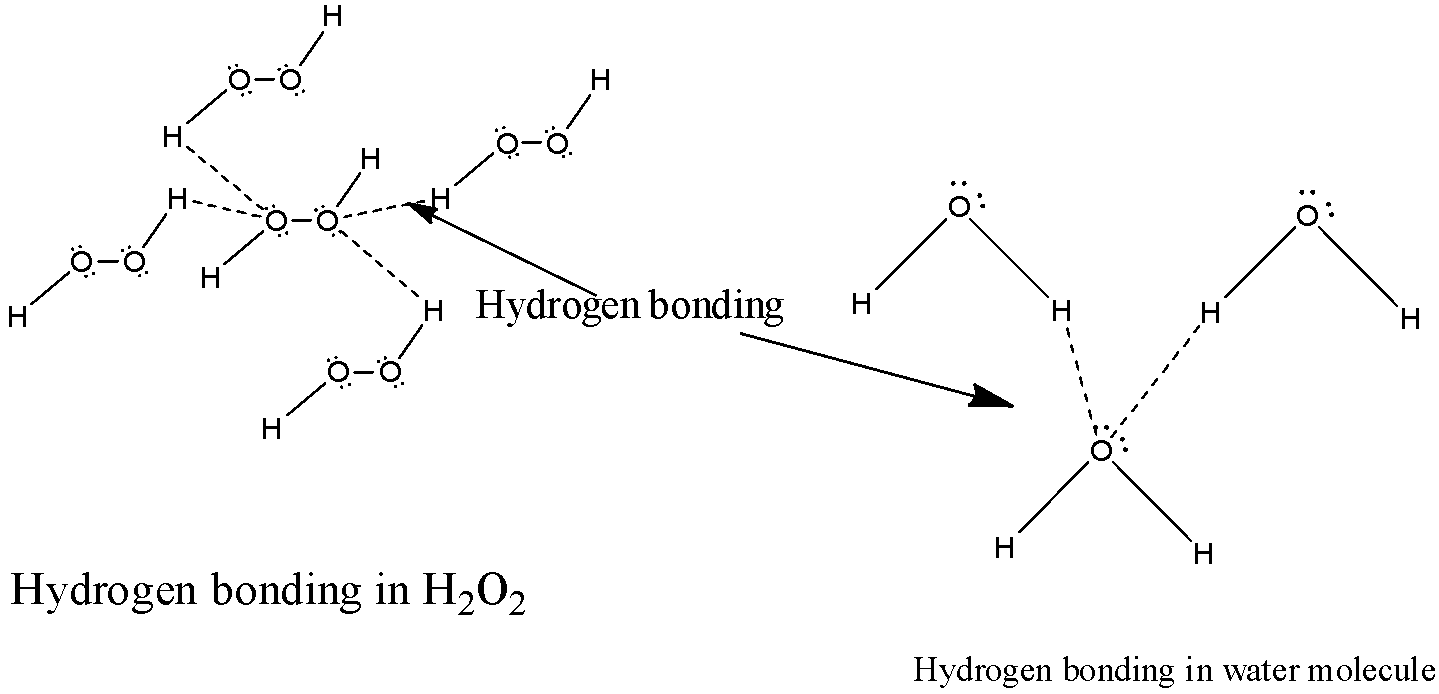
4) In the water molecule there is only one oxygen atom present which means there can be only two hydrogen bonds possible between the electronegative oxygen atom and the hydrogen atom.
5) Therefore, In ${H_2}{O_2}$ molecules the degree of hydrogen bonding is more than in water which shows option B as the correct choice.
Therefore, the correct option is B.
Note:
The Intermolecular forces are present between the atoms of two molecules. The bonds formed by hydrogen bonding are stronger than the dipole-dipole and dispersion forces and they are weaker bonds than the covalent and ionic bonds.
Complete answer:
1) First of all we will learn about the concept of hydrogen bonding. Hydrogen bonding is the intermolecular type of bonding which is an intermolecular force.
2) The intermolecular force forms a special type of dipole-dipole attraction between a hydrogen atom bond and a strongly electronegative atom of another molecule present nearby which also possesses a lone pair of electrons.
3) Now let us analyze the structure ${H_2}{O_2}$ in which there are two oxygen molecules present. Each oxygen molecule will form two hydrogen bonds with two molecules of ${H_2}{O_2}$ which will result in the formation of four hydrogen bonds in a structure of hydrogen peroxide. The structure of hydrogen bonding ${H_2}{O_2}$ will be as below,

4) In the water molecule there is only one oxygen atom present which means there can be only two hydrogen bonds possible between the electronegative oxygen atom and the hydrogen atom.
5) Therefore, In ${H_2}{O_2}$ molecules the degree of hydrogen bonding is more than in water which shows option B as the correct choice.
Therefore, the correct option is B.
Note:
The Intermolecular forces are present between the atoms of two molecules. The bonds formed by hydrogen bonding are stronger than the dipole-dipole and dispersion forces and they are weaker bonds than the covalent and ionic bonds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




