
In Hydrogen spectrum, the spectral line of Balmer series having lowest wavelength is:
A.${H_\alpha } - line$
B.${H_\beta } - line$
C.${H_\gamma } - line$
D.${H_\delta } - line$
Answer
565.2k+ views
Hint:We know that orbits have a different energy. These orbitals are K, L, M, etc and these have n values as 1, 2,3 …..etc. Some atoms have only 1 electron in its valence cell. It absorbs energy and jumps to higher energy levels. 2 of the examples can be hydrogen and sodium. Hydrogen has only 1 electron. Therefore, it gains energy and jumps to higher energy levels. It then comes back to the ground energy level or lower energy level and the wavelength of energy emitted during this is called the hydrogen spectrum
Complete step by step answer:
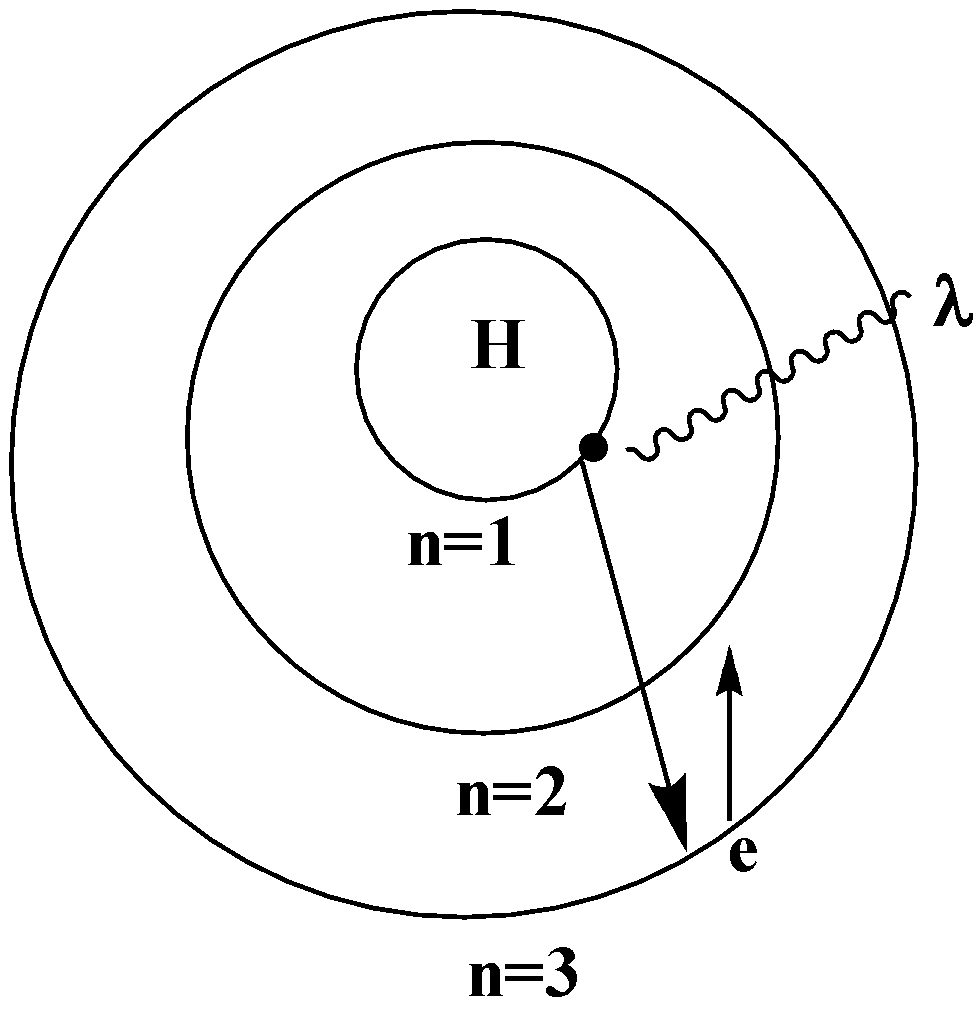
When an electron in a hydrogen atom jumps from a higher energy level to a lower energy level, the difference in energy of the 2 energy levels is emitted as a radiation of wavelength $\lambda $
There are different spectral series of the hydrogen atom. They are the Lyman series, Balmer series, Paschen series, etc
When the electron jumps from the outer orbit or the higher energy orbit to the second orbit ($n = 2$), the spectral lines formed are the Balmer series. The wavelength lies in the visible region.
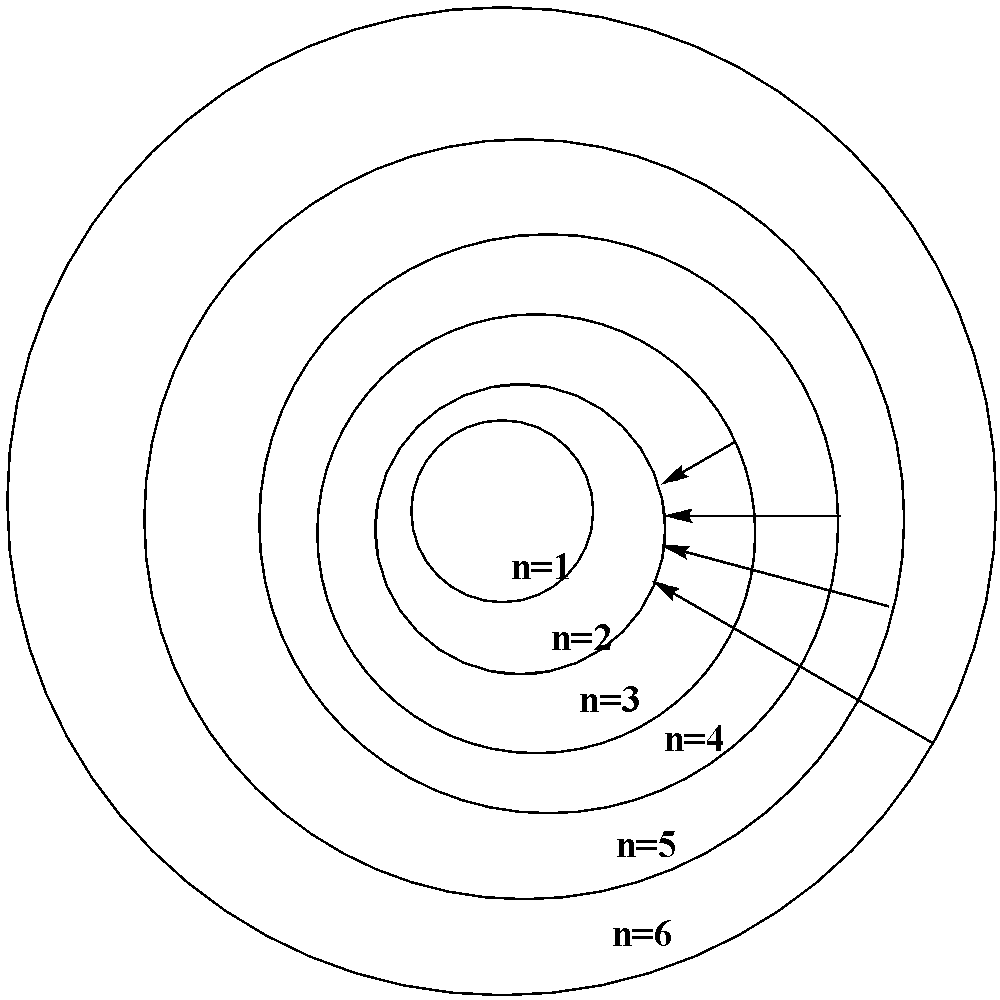
The first line in the series where the electron jumps from ${{n = 3}}$ to $n = 2$ is called the ${{{H}}_{{\alpha }}}{{ line}}$.
The second line in the series where the electron jumps from $n = 4$ to $n = 2$ is the ${H_\beta }{{ line}}$ and so on.
The wavenumber can be calculated by the Rydberg formula in which
$wavenumber = {R_H}\left[ {\dfrac{1}{{{2^2}}} - \dfrac{1}{{{n^2}}}} \right]$
${R_H}$ is the Rydberg constant.
We know that $Wavenumber = \dfrac{1}{\lambda }$ , wavenumber is inversely proportional to the wavelength.
From the above formula, we can say that as n increases the wavenumber also increases. Since wavelength is inversely proportional to the wavenumber, the wavelength decreases.
Here for $H\alpha (n = 3)$ , ${H_\beta }(n = 4)$ , ${H_\gamma }(n = 5)$ and ${H_\delta }(n = 6)$.
So we can say that wavelength is the lowest for ${H_\delta }(n = 6)$
The correct answer is option D.
Note:
The Lyman series is when the electrons from the outer orbital jumps to the $n = 1$ energy level. The wavelength emitted is in the ultraviolet region. The Paschen series is the line spectra obtained when the electron jumps from the outer orbitals to the $n = 3$ orbital of the Hydrogen atom. This is in the infrared region.

Complete step by step answer:
When an electron in a hydrogen atom jumps from a higher energy level to a lower energy level, the difference in energy of the 2 energy levels is emitted as a radiation of wavelength $\lambda $
There are different spectral series of the hydrogen atom. They are the Lyman series, Balmer series, Paschen series, etc
When the electron jumps from the outer orbit or the higher energy orbit to the second orbit ($n = 2$), the spectral lines formed are the Balmer series. The wavelength lies in the visible region.

The first line in the series where the electron jumps from ${{n = 3}}$ to $n = 2$ is called the ${{{H}}_{{\alpha }}}{{ line}}$.
The second line in the series where the electron jumps from $n = 4$ to $n = 2$ is the ${H_\beta }{{ line}}$ and so on.
The wavenumber can be calculated by the Rydberg formula in which
$wavenumber = {R_H}\left[ {\dfrac{1}{{{2^2}}} - \dfrac{1}{{{n^2}}}} \right]$
${R_H}$ is the Rydberg constant.
We know that $Wavenumber = \dfrac{1}{\lambda }$ , wavenumber is inversely proportional to the wavelength.
From the above formula, we can say that as n increases the wavenumber also increases. Since wavelength is inversely proportional to the wavenumber, the wavelength decreases.
Here for $H\alpha (n = 3)$ , ${H_\beta }(n = 4)$ , ${H_\gamma }(n = 5)$ and ${H_\delta }(n = 6)$.
So we can say that wavelength is the lowest for ${H_\delta }(n = 6)$
The correct answer is option D.
Note:
The Lyman series is when the electrons from the outer orbital jumps to the $n = 1$ energy level. The wavelength emitted is in the ultraviolet region. The Paschen series is the line spectra obtained when the electron jumps from the outer orbitals to the $n = 3$ orbital of the Hydrogen atom. This is in the infrared region.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




