
In hyponitrous acid, the number of hydroxyl group present are:
(A). $1$
(B). $2$
(C). $3$
(D). $4$
Answer
576k+ views
Hint: There are two possible structure of acid, Tans and cis Trans-Hyponitrous acid forms with crystals that are explosive when dry. In solution, it is a weak acid $\left( {P{K_{a1}} = 7.21,P{K_{a2}} = 11.54} \right)$ and decomposed to laughing gas and water with a half lifetime of $16$ days at $25^\circ C$ at pH $1 - 3$ .
Complete answer:
Hyponitrous acid is chemical compound with formula ${H_2}{N_2}{O_2}$ or $HON = NOH$ . it is an isomer tautomer of nitramide, ${H_2}N - N{O_2}$
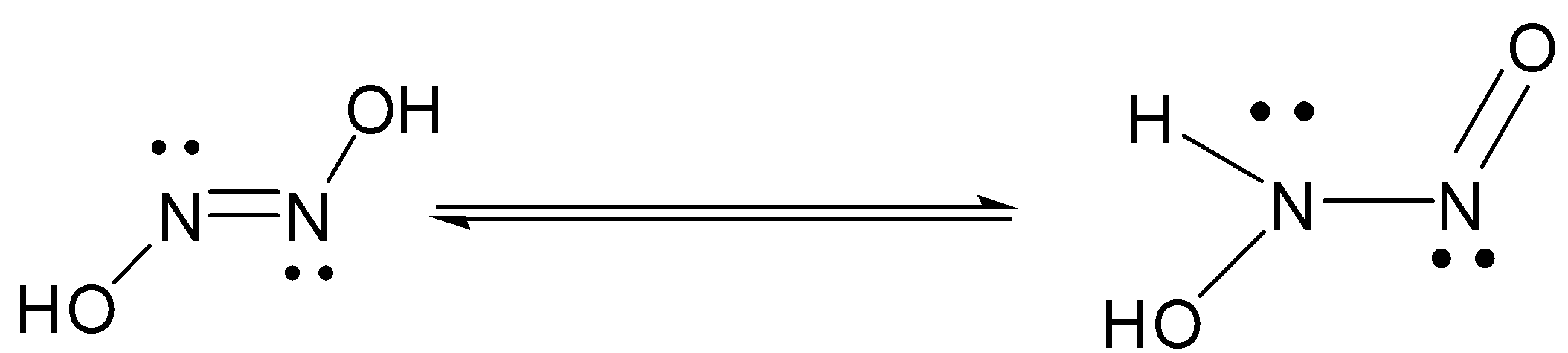
The number of hydroxyl groups present are two. The preparation of hyponitrous acid ( trans isomer ) can be done with silver hyponitrite and anhydrous $HCl$ in ether.
$A{g_2}{N_2}{O_2} + 2HCl \to {H_2}{N_2}{O_2} + 2AgCl$
Ferrous hydroxide will also reduce nitrates to hyponitrites.
Pure ferrous sulphate is precipitated with milk of lime, and to the cooled mixture is added $1$ mol of $NaN{O_3}$ for each $10$ moles of $FeS{O_4}$ . The hyponitrite is precipitated with silver nitrate.
A solution of $NaN{O_2}$ is added to an answer of $N{H_2}OH - {H_2}S{O_4}$ . The mixture is heated quickly to $60^\circ C$ and $AgN{O_3}$ is added at once. This method does not give good yields, but it proves that acid may be a dioxime. It proceeds as follows-
$HO - N{H_2} + O = N - OH \to HO - N = N - OH + {H_2}O$
Therefore, option B is correct.
Additional Information:
Salt of Hyponitrous acid is soluble in water and hydrolyse and gives an alkaline solution . Whereas normal salts of other bases are less soluble .
In the dry state it decomposes at $100^\circ C$ , giving $AgN{O_3}$ and it explodes at $150^\circ C$ .It is not decomposed by alkalies. It reacts with alkyl iodides, giving alkyl hypritrites.
Note:
It was shown by Divers in $1871$ , the sodium nitrate reduced with sodium amalgam gives a salt $NaNO$ (empirical composition) , the acid being an isomer of nitramide.
Since the discovery of hyponitrous acid by Dr. Divers , no new oxyacid of nitrogen has been described .
Complete answer:
Hyponitrous acid is chemical compound with formula ${H_2}{N_2}{O_2}$ or $HON = NOH$ . it is an isomer tautomer of nitramide, ${H_2}N - N{O_2}$

The number of hydroxyl groups present are two. The preparation of hyponitrous acid ( trans isomer ) can be done with silver hyponitrite and anhydrous $HCl$ in ether.
$A{g_2}{N_2}{O_2} + 2HCl \to {H_2}{N_2}{O_2} + 2AgCl$
Ferrous hydroxide will also reduce nitrates to hyponitrites.
Pure ferrous sulphate is precipitated with milk of lime, and to the cooled mixture is added $1$ mol of $NaN{O_3}$ for each $10$ moles of $FeS{O_4}$ . The hyponitrite is precipitated with silver nitrate.
A solution of $NaN{O_2}$ is added to an answer of $N{H_2}OH - {H_2}S{O_4}$ . The mixture is heated quickly to $60^\circ C$ and $AgN{O_3}$ is added at once. This method does not give good yields, but it proves that acid may be a dioxime. It proceeds as follows-
$HO - N{H_2} + O = N - OH \to HO - N = N - OH + {H_2}O$
Therefore, option B is correct.
Additional Information:
Salt of Hyponitrous acid is soluble in water and hydrolyse and gives an alkaline solution . Whereas normal salts of other bases are less soluble .
In the dry state it decomposes at $100^\circ C$ , giving $AgN{O_3}$ and it explodes at $150^\circ C$ .It is not decomposed by alkalies. It reacts with alkyl iodides, giving alkyl hypritrites.
Note:
It was shown by Divers in $1871$ , the sodium nitrate reduced with sodium amalgam gives a salt $NaNO$ (empirical composition) , the acid being an isomer of nitramide.
Since the discovery of hyponitrous acid by Dr. Divers , no new oxyacid of nitrogen has been described .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




