
In $ICl_4^ \ominus $ the shape is square planar. The number of bond pair-lone repulsion at $90^\circ $ are:
(A). $6$
(B). $8$
(C). $12$
(D). $4$
Answer
569.1k+ views
Hint:
We know that $ICl_4^ \ominus $ is $A{B_4}{E_2}$ type molecule and it shows:
Electronic Structure: $A{B_4}{E_2}$
Electronic Geometry: Octahedral
Hybridization of central atom is $s{p^3}{d^2}$
Complete step by step answer:
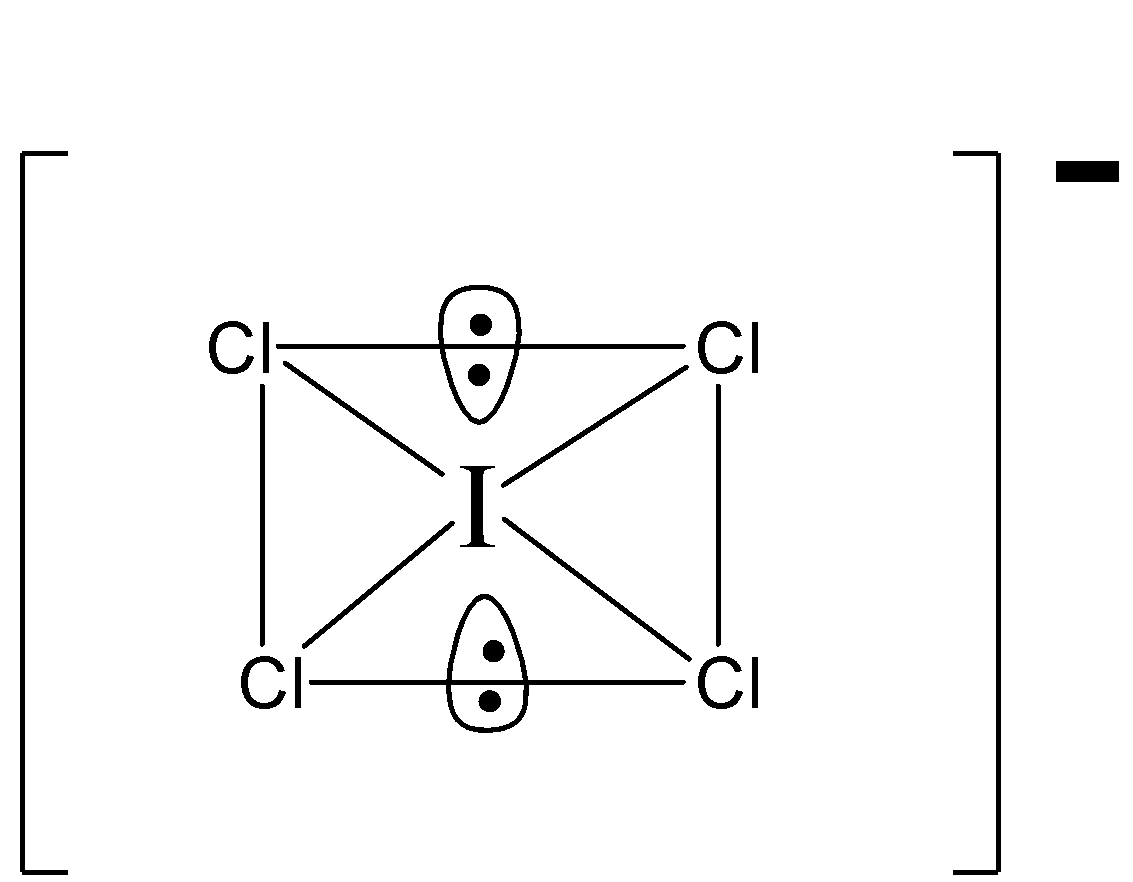
There are two lone pairs of electrons which are perpendicular to the square plane. In the square plane there are $8$ lone pair electrons.
Thus, repulsion at $90^\circ = 8$
Therefore, the correct option is (B) $8$.
Additional Information:
In the lewis structure of $ICl_4^ \ominus $ there are total $36$ valence electrons.
Since Iodine $\left( I \right)$ is below period $3$ on the periodic table, it can have more than $8$ electrons. In the lewis structure of $ICl_4^ \ominus $ the iodine atom has $12$ valence electrons.
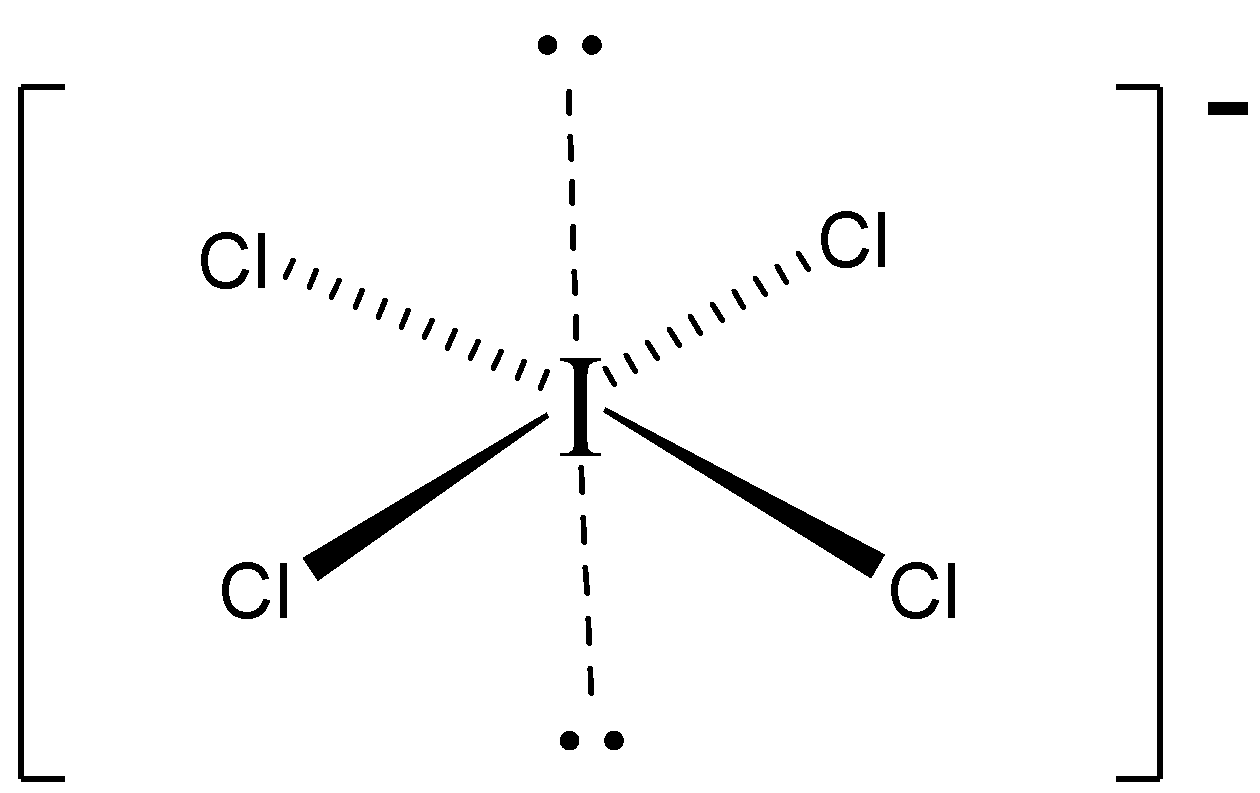
$3 - D$ structure.
Important Points:
In $ICl_4^ \ominus $ lewis structure, Iodine $\left( I \right)$ has the least electronegativity and goes in the center of lewis structure.
The $ICl_4^ \ominus $ lewis structure you’ll need to put more than eight electrons on the iodine atom.
In the lewis structure for $ICl_4^ \ominus $, there are a total of $36$ electrons.
Note: Note that you should put $ICl_4^ \ominus $ lewis structure in brackets with $ - 1$ charge outside to show that it is an ion with negative one charge.
We know that $ICl_4^ \ominus $ is $A{B_4}{E_2}$ type molecule and it shows:
Electronic Structure: $A{B_4}{E_2}$
Electronic Geometry: Octahedral
Hybridization of central atom is $s{p^3}{d^2}$
Complete step by step answer:
There are two lone pairs of electrons which are perpendicular to the square plane. In the square plane there are $8$ lone pair electrons.
Thus, repulsion at $90^\circ = 8$

Therefore, the correct option is (B) $8$.
Additional Information:
In the lewis structure of $ICl_4^ \ominus $ there are total $36$ valence electrons.
Since Iodine $\left( I \right)$ is below period $3$ on the periodic table, it can have more than $8$ electrons. In the lewis structure of $ICl_4^ \ominus $ the iodine atom has $12$ valence electrons.
$3 - D$ structure.

Important Points:
In $ICl_4^ \ominus $ lewis structure, Iodine $\left( I \right)$ has the least electronegativity and goes in the center of lewis structure.
The $ICl_4^ \ominus $ lewis structure you’ll need to put more than eight electrons on the iodine atom.
In the lewis structure for $ICl_4^ \ominus $, there are a total of $36$ electrons.
Note: Note that you should put $ICl_4^ \ominus $ lewis structure in brackets with $ - 1$ charge outside to show that it is an ion with negative one charge.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




