
In \[{\mathbf{HONO}}\], number of free lone pairs on one \[{\mathbf{O}}\] atom in the above given compound are:
Answer
570.3k+ views
Hint: By taking help of Lewis structure we can determine the lone pairs of the given compound. The Lewis structure shows the bonding between atoms of a particle and the lone pairs of electrons that might exist within the molecule.
Complete step by step solution: With the help of Lewis structure, we can determine the number of free lone pairs on one O atom in the compound. By Counting the total number of valence electrons of the hydrogen, nitrogen and oxygen atoms. Or we can say the valence shell configuration and hence valence electrons of these atoms in
Hydrogen \[H\left( {1{s^1}} \right) = {\text{ }}1{\text{ }}electron\]
Nitrogen \[N\left( {2{s^2}2{p^3}} \right){\text{ }} = {\text{ }}5{\text{ }}electrons\]
Oxygen \[O{\text{ }}\left( {2{s^2}2{p^4}} \right){\text{ }} = {\text{ }}2 \times 6{\text{ }}electrons\] (Because 2 oxygen atoms are there in HONO molecule).
so, total valence shell electrons in HONO molecules\[ = {\text{ }}1 + 5 + {\text{ }}12{\text{ }} = {\text{ }}18{\text{ }}electrons\].
The Lewis structure of HONO is written as the central atom will be the one which has maximum valency. Thus, nitrogen is the central atom because it shows maximum valence 5 electrons which is greater than maximum and 2 of oxygen and 1 of hydrogen. Therefore, the skeletal Structure is HONO.
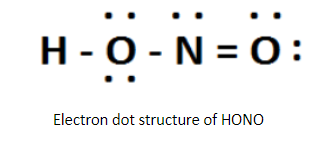
By sketching a single bond between H and O, O and N, N and 0 in a straight line along with Completing the octet on each atom. Thus, remaining unshared valence electrons establish as lone pairs. So, there will be a double bond between nitrogen and one oxygen atom to minimize lone pairs.
Thus, the required answer is 2.
So, the correct answer is 2 lone pairs.
Note: it should be noted that after adding the shared pairs of electron pairs for all the single bonds in a molecule, the remaining electron pairs are either used for multiple bonding or remain as the free lone pairs. In compound HONO the number of free lone pairs on O atom are 2 because O has 6 valence electrons out of which 2 are employed in forming 2 covalent bonds and other remaining 4 electrons are present as 2 lone pairs.
Complete step by step solution: With the help of Lewis structure, we can determine the number of free lone pairs on one O atom in the compound. By Counting the total number of valence electrons of the hydrogen, nitrogen and oxygen atoms. Or we can say the valence shell configuration and hence valence electrons of these atoms in
Hydrogen \[H\left( {1{s^1}} \right) = {\text{ }}1{\text{ }}electron\]
Nitrogen \[N\left( {2{s^2}2{p^3}} \right){\text{ }} = {\text{ }}5{\text{ }}electrons\]
Oxygen \[O{\text{ }}\left( {2{s^2}2{p^4}} \right){\text{ }} = {\text{ }}2 \times 6{\text{ }}electrons\] (Because 2 oxygen atoms are there in HONO molecule).
so, total valence shell electrons in HONO molecules\[ = {\text{ }}1 + 5 + {\text{ }}12{\text{ }} = {\text{ }}18{\text{ }}electrons\].
The Lewis structure of HONO is written as the central atom will be the one which has maximum valency. Thus, nitrogen is the central atom because it shows maximum valence 5 electrons which is greater than maximum and 2 of oxygen and 1 of hydrogen. Therefore, the skeletal Structure is HONO.
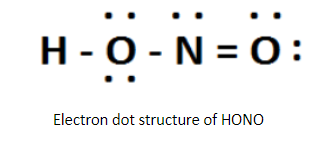
By sketching a single bond between H and O, O and N, N and 0 in a straight line along with Completing the octet on each atom. Thus, remaining unshared valence electrons establish as lone pairs. So, there will be a double bond between nitrogen and one oxygen atom to minimize lone pairs.
Thus, the required answer is 2.
So, the correct answer is 2 lone pairs.
Note: it should be noted that after adding the shared pairs of electron pairs for all the single bonds in a molecule, the remaining electron pairs are either used for multiple bonding or remain as the free lone pairs. In compound HONO the number of free lone pairs on O atom are 2 because O has 6 valence electrons out of which 2 are employed in forming 2 covalent bonds and other remaining 4 electrons are present as 2 lone pairs.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




