
In ${P_4}{O_{10}}$ the:
(A) Second bond in $P = O$ is formed by $p\pi - d\pi $ back bonding
(B) $P = O$ bond is formed by $p\pi - p\pi $ bonding
(C) $P = O$ bond is formed by $d\pi - d\pi $ bonding
(D) $P = O$ bond is formed by $d\pi - d\pi - 3\sigma $ back bonding
Answer
586.2k+ views
Hint:${P_4}{O_{10}}$ (phosphorus Pentoxide) is a chemical compound. This while crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.
Complete step by step answer:
In ${P_4}{O_{10}}$, the terminal $P - O$ bonds are formed by $p\pi - d\pi $ back bonding. This bonding results in the formation of a coordination bond resulting from an overlap of the p-orbitals of oxygen with empty $d\pi $ orbitals of phosphorus. Here, phosphorus atoms donate one pair of electrons resulting in $\pi $ back bonding.
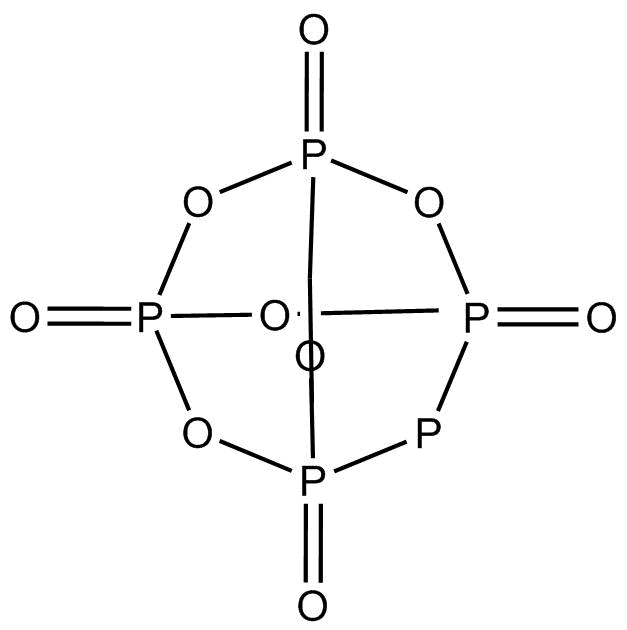
The molecules are held together in a hexagonal lattice by weak Vander Waals forces.
This structure contains $6P - O - P$ bonds where the hybridization of oxygen is $s{p^3}$. Another $4$ oxygens are attached to each phosphorus by $P = O$ where hybridization of oxygen is $s{p^2}$. All the $10$ oxygen contains $20$lone pairs ($2$ lone pairs each).
Preparation: ${P_4} + 5{O_2} \to {P_4}{O_{10}}$.
Hence, option B is the answer.
Note:
${P_4}{O_{10}}$ is called pentoxide because of the oxidation state of phosphorus in the compound. The name of phosphorus pentoxide itself suggests that the name is related to phosphorus. The naming of any compound is done using its empirical formula. Since, the empirical formula of ${P_4}{O_{10}}$ is ${P_2}{O_5}$, it is called phosphorus pentoxide.
Complete step by step answer:
In ${P_4}{O_{10}}$, the terminal $P - O$ bonds are formed by $p\pi - d\pi $ back bonding. This bonding results in the formation of a coordination bond resulting from an overlap of the p-orbitals of oxygen with empty $d\pi $ orbitals of phosphorus. Here, phosphorus atoms donate one pair of electrons resulting in $\pi $ back bonding.

The molecules are held together in a hexagonal lattice by weak Vander Waals forces.
This structure contains $6P - O - P$ bonds where the hybridization of oxygen is $s{p^3}$. Another $4$ oxygens are attached to each phosphorus by $P = O$ where hybridization of oxygen is $s{p^2}$. All the $10$ oxygen contains $20$lone pairs ($2$ lone pairs each).
Preparation: ${P_4} + 5{O_2} \to {P_4}{O_{10}}$.
Hence, option B is the answer.
Note:
${P_4}{O_{10}}$ is called pentoxide because of the oxidation state of phosphorus in the compound. The name of phosphorus pentoxide itself suggests that the name is related to phosphorus. The naming of any compound is done using its empirical formula. Since, the empirical formula of ${P_4}{O_{10}}$ is ${P_2}{O_5}$, it is called phosphorus pentoxide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




