
In Pyrrole the electron density is maximum on:
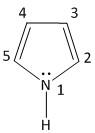
A. \[2{\text{ }}and{\text{ }}4\]
B. \[2{\text{ }}and{\text{ }}5\]
C. \[2{\text{ }}and{\text{ }}3\]
D. \[3{\text{ }}and{\text{ }}4\]

Answer
563.4k+ views
Hint:Pyrrole is a five membered heterocyclic compound. The electron density on the carbon atoms is dependent on the movement of the non-bonding electrons of nitrogen inside the ring.
Complete step by step answer: Pyrrole belongs to the heterocyclic family of compounds as it contains hetero atom in the form of nitrogen in a ring skeleton. The chemical formula of pyrrole is \[{C_4}{H_4}NH\].
-Pyrrole appears a volatile colourless liquid which generates brown colour on exposure to air. Pyrrole moiety is an important heterocycle which is the component of various natural macromolecules. It is obtained as a distillation product like furan and thiophene.
-The electrons current of the pyrrole is delocalized in the ring. The electron density of nitrogen in a pyrrole ring is in resonance with the pi electrons of the ring. It is written in the form of following structures:
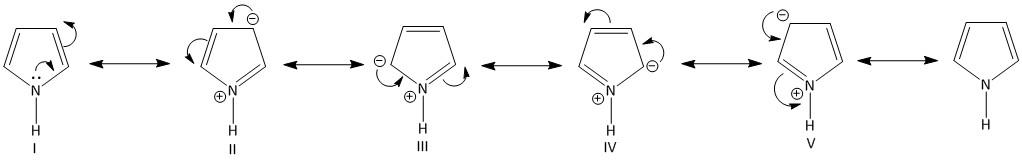
All the five structures are termed as the resonating structures of pyrrole. Each of the structures contributes equally to the resonance energy of the molecule. The resonating structures indicate that the nitrogen carries positive charge and the four carbon atoms carries negative charge.
Of the five resonating structures the charge separation is less in structure III and IV. However the charge separation is more in II and V. This results in more stability to the structures III and IV, where the negative charge is present at the \[C - 2\] and \[C - 5\] carbon atoms.
Hence the option B is the correct answer, i.e. the electron density is maximum and \[2{\text{ }}and{\text{ }}5\] .
Note:
Due to more electron density at the \[C - 2\] and \[C - 5\] position of the pyrrole the electrophilic substitution is more favored at these positions. Thus the nitration or sulfonation or halogenations of pyrrole occurs in the \[2\]-position.
Complete step by step answer: Pyrrole belongs to the heterocyclic family of compounds as it contains hetero atom in the form of nitrogen in a ring skeleton. The chemical formula of pyrrole is \[{C_4}{H_4}NH\].
-Pyrrole appears a volatile colourless liquid which generates brown colour on exposure to air. Pyrrole moiety is an important heterocycle which is the component of various natural macromolecules. It is obtained as a distillation product like furan and thiophene.
-The electrons current of the pyrrole is delocalized in the ring. The electron density of nitrogen in a pyrrole ring is in resonance with the pi electrons of the ring. It is written in the form of following structures:

All the five structures are termed as the resonating structures of pyrrole. Each of the structures contributes equally to the resonance energy of the molecule. The resonating structures indicate that the nitrogen carries positive charge and the four carbon atoms carries negative charge.
Of the five resonating structures the charge separation is less in structure III and IV. However the charge separation is more in II and V. This results in more stability to the structures III and IV, where the negative charge is present at the \[C - 2\] and \[C - 5\] carbon atoms.
Hence the option B is the correct answer, i.e. the electron density is maximum and \[2{\text{ }}and{\text{ }}5\] .
Note:
Due to more electron density at the \[C - 2\] and \[C - 5\] position of the pyrrole the electrophilic substitution is more favored at these positions. Thus the nitration or sulfonation or halogenations of pyrrole occurs in the \[2\]-position.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




