
In pyrrole the electron density is maximum on which number of atoms?
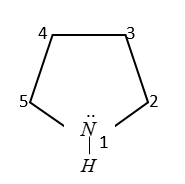
(A) 2 and 3
(B) 3 and 4
(C) 2 and 4
(D) 2 and 5

Answer
582.3k+ views
Hint: In conjugation system $\pi $electrons and lone pair of electrons are in conjugation with each other. $\pi $electrons are delocalized and constantly moving on aromatic rings.
Step by step answer: Pyrol is an aromatic compound. Let us prove that pyrrole is an aromatic compound.
These compounds should have the following properties.
(i) Each carbon atom should be planer ($s{p^2}$ hybridization)
Here C-atoms at 2, 3, 4, 5 positions are $s{p^2}$ hybridized
(ii) There should be a conjugated system.
Conjugated system has $\pi $-bond -$\sigma $-bond-lone pair of electrons alternately.
This system also presents in pyrol.
(iii) There should be $(4n + 2)\pi $electrons present
Where $n = 0,1,2,3 - - - - - $
i.e., $2\pi $ electrons, $6\pi $ electrons, $10\pi $ electrons and so on.
$\therefore $ Pyrol is an aromatic compound.
In aromatic compounds $\pi $ electrons are delocalized.
This delocalization of $\pi $ electrons is with a lone pair of electrons.
$\therefore $ In pyrol, electron density is maximum at 2nd and $5m$ carbon.
We can observe this from its Resonance structure. These structures are stable, other structures are not stable.
Therefore, from the above explanation the correct option is (D) 2 and 5.
Note: Electron density of aromatic compound can be determined by deallocation of $\pi $electrons and lone pair of electrons on aromatic rings.
Step by step answer: Pyrol is an aromatic compound. Let us prove that pyrrole is an aromatic compound.
These compounds should have the following properties.
(i) Each carbon atom should be planer ($s{p^2}$ hybridization)
Here C-atoms at 2, 3, 4, 5 positions are $s{p^2}$ hybridized
(ii) There should be a conjugated system.
Conjugated system has $\pi $-bond -$\sigma $-bond-lone pair of electrons alternately.
This system also presents in pyrol.
(iii) There should be $(4n + 2)\pi $electrons present
Where $n = 0,1,2,3 - - - - - $
i.e., $2\pi $ electrons, $6\pi $ electrons, $10\pi $ electrons and so on.
$\therefore $ Pyrol is an aromatic compound.
In aromatic compounds $\pi $ electrons are delocalized.
This delocalization of $\pi $ electrons is with a lone pair of electrons.

$\therefore $ In pyrol, electron density is maximum at 2nd and $5m$ carbon.
We can observe this from its Resonance structure. These structures are stable, other structures are not stable.
Therefore, from the above explanation the correct option is (D) 2 and 5.
Note: Electron density of aromatic compound can be determined by deallocation of $\pi $electrons and lone pair of electrons on aromatic rings.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




