
In silica $(Si{O_2})$, each silicon atom is bonded to :
A. two oxygen atoms
B. four oxygen atoms
C. one silicon and two oxygen atoms
D. one silicon and four oxygen atoms
Answer
598.5k+ views
Hint: Silica is a white colourless compound. It is found as quartz, sand, flint and many other minerals. Silicon is represented by the symbol Si and the atomic number is 14. It is the fourteen element on the periodic table. Silica is the most abundant mineral that can be easily found in the crust of the earth. Colloidal silica acts as a fining agent for wine, juice and beer. It is also very important for semiconductors.
Complete answer:
Silica is a compound of silicon and oxygen. It is naturally found in many crystalline forms ,quartz is one of them. It is also known as silicon dioxide. In $Si{O_2}$ Si atom is surrounded by four oxygen atoms in tetrahedral arrangement. In 3-D structure each silicon atom is covalently bonded in tetrahedral manner to 4 oxygen atoms. The following structure shows that in silica, Si is bonded to four oxygen atoms.
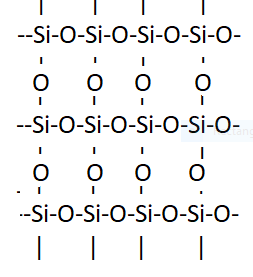 Structure of silica ( $Si{O_2}$)
Structure of silica ( $Si{O_2}$)
So here option B is correct as now we know that In silica $(Si{O_2})$, each silicon atom is bonded to four oxygen atoms.
Note: Here we have learned about silica, its properties and uses. Silica has a number of different crystalline forms. Silicon-oxygen bond lengths vary between different crystals of $Si{O_2}$. Quartz is the only form stable at the earth surface. The different forms of silica can be converted from one form to another by heating or changing pressure. Silica is mostly used in the construction industry .It is used in the production of concrete. Its high melting point makes it usable in many industries such as metallic components in engineering.
Complete answer:
Silica is a compound of silicon and oxygen. It is naturally found in many crystalline forms ,quartz is one of them. It is also known as silicon dioxide. In $Si{O_2}$ Si atom is surrounded by four oxygen atoms in tetrahedral arrangement. In 3-D structure each silicon atom is covalently bonded in tetrahedral manner to 4 oxygen atoms. The following structure shows that in silica, Si is bonded to four oxygen atoms.
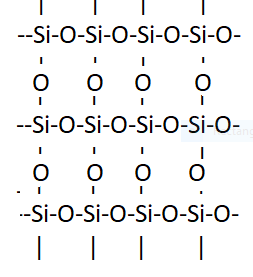
So here option B is correct as now we know that In silica $(Si{O_2})$, each silicon atom is bonded to four oxygen atoms.
Note: Here we have learned about silica, its properties and uses. Silica has a number of different crystalline forms. Silicon-oxygen bond lengths vary between different crystals of $Si{O_2}$. Quartz is the only form stable at the earth surface. The different forms of silica can be converted from one form to another by heating or changing pressure. Silica is mostly used in the construction industry .It is used in the production of concrete. Its high melting point makes it usable in many industries such as metallic components in engineering.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




