
In $TeC{{l}_{4}}$, the central atom tellurium involves:
(A) $s{{p}^{3}}$ hybridisation
(B) $s{{p}^{3}}d$ hybridisation
(C) $s{{p}^{3}}{{d}^{2}}$ hybridisation
(D) $ds{{p}^{2}}$ hybridisation
Answer
583.8k+ views
Hint: To find out the hybridisation here approach using the valence bond theory. Start by writing down the electron configuration of the central atom. When a chlorine group is attached, the central atoms will pair up and the chlorine electrons will take up the remaining p and d-orbital. This will give us the required hybridisation.
Complete step by step answer:
In the valence bond theory, the atomic orbitals overlap with other atomic orbitals to form a molecule and thus creating new hybrid orbitals. This is known as the phenomenon of hybridisation.
The compound given to us is $TeC{{l}_{4}}$. To find its hybridisation, firstly let us write down its electronic configuration.
Tellurium belongs to group 16 and its atomic number is 52. So, we can write its electronic configuration as- $\left( Kr \right)4{{d}^{10}}5{{s}^{2}}5{{p}^{4}}$.
Tellurium has 2 electrons in s-orbital and 4 electrons in p-orbital in neutral state. When chlorine is attached to it, it is in +4 oxidation state and one electron from the p electron moves to the outer d-orbital which gives us an outer d-orbital complex.
We can draw the orbital diagram as:
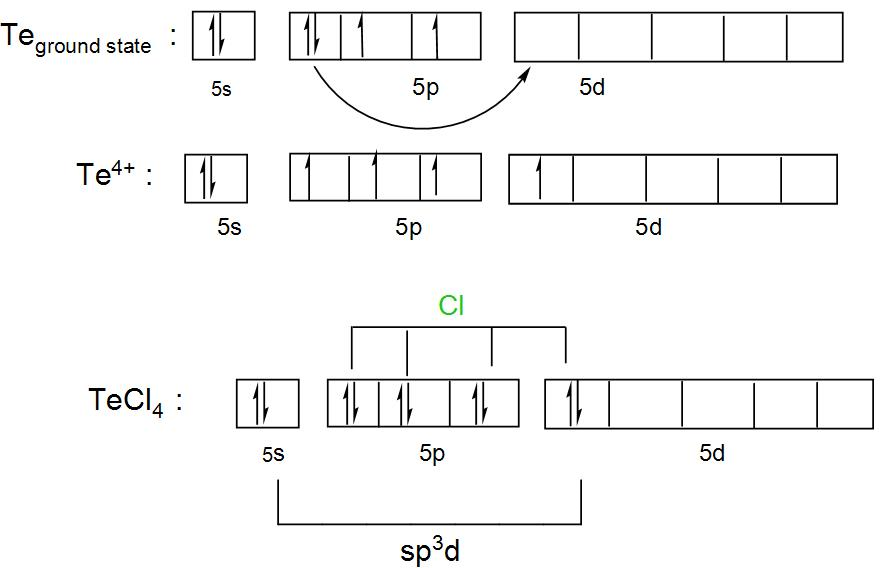
We can understand from the above discussion and the diagram that its hybridisation is $s{{p}^{3}}d$ and it forms an outer orbital complex as the chlorine atoms will occupy the outer orbitals of the tellurium atom.
Therefore, the correct answer is option (B) $s{{p}^{3}}d$ hybridisation.
Note: There are different ways to calculate hybridisation. We can calculate it from CFT and steric number concepts too besides the VBT concept like we did in the above question.
However, there are certain limitations to the VBT theory.
- The preferred geometry and colour of the complex cannot be explained.
- The distortion of the shape of a complex from regular geometry cannot be explained.
- The geometry of a complex cannot be predicted correctly using the magnetic moment data always.
- Classification that inner orbital complexes are covalent and outer orbital complexes are ionic is misleading.
Complete step by step answer:
In the valence bond theory, the atomic orbitals overlap with other atomic orbitals to form a molecule and thus creating new hybrid orbitals. This is known as the phenomenon of hybridisation.
The compound given to us is $TeC{{l}_{4}}$. To find its hybridisation, firstly let us write down its electronic configuration.
Tellurium belongs to group 16 and its atomic number is 52. So, we can write its electronic configuration as- $\left( Kr \right)4{{d}^{10}}5{{s}^{2}}5{{p}^{4}}$.
Tellurium has 2 electrons in s-orbital and 4 electrons in p-orbital in neutral state. When chlorine is attached to it, it is in +4 oxidation state and one electron from the p electron moves to the outer d-orbital which gives us an outer d-orbital complex.
We can draw the orbital diagram as:

We can understand from the above discussion and the diagram that its hybridisation is $s{{p}^{3}}d$ and it forms an outer orbital complex as the chlorine atoms will occupy the outer orbitals of the tellurium atom.
Therefore, the correct answer is option (B) $s{{p}^{3}}d$ hybridisation.
Note: There are different ways to calculate hybridisation. We can calculate it from CFT and steric number concepts too besides the VBT concept like we did in the above question.
However, there are certain limitations to the VBT theory.
- The preferred geometry and colour of the complex cannot be explained.
- The distortion of the shape of a complex from regular geometry cannot be explained.
- The geometry of a complex cannot be predicted correctly using the magnetic moment data always.
- Classification that inner orbital complexes are covalent and outer orbital complexes are ionic is misleading.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




