
In the cubic close packing, this unit cell has:
A. 4 tetrahedral voids each of which is shared by four adjacent unit cells.
B. 4 tetrahedral voids within the cell.
C. 8 tetrahedral voids each of which is shared by four adjacent unit cells.
D. 8 tetrahedral voids within the unit cells.
Answer
588.6k+ views
Hint: The tiniest group of atoms which represent the overall symmetry of the crystal and by using unit cells the entire lattice of the cube is built up by repeating in three dimensions. Cubic close packing (CCP) is similar to face-centered cubic (fcc) structure.
Complete step by step answer:
-If there is an ‘n’ number of unit cells then the number of voids is double the number of unit cells means ‘2n’.
-The void in a cube has a smaller volume and the coordination number for the tetrahedral void is four.
-As cubic close packing is similar to face-centered cubic (fcc) then the number of atoms in a fcc structure is 4 each to one unit cell
-We know that the number of tetrahedral voids = 2n
-Thus the total number of tetrahedral voids = 2 (4) = 8.
-Therefore 8 tetrahedral voids are present within the unit cell.
- The representation of the 8 tetrahedral voids within the unit cells can see in the following structure.
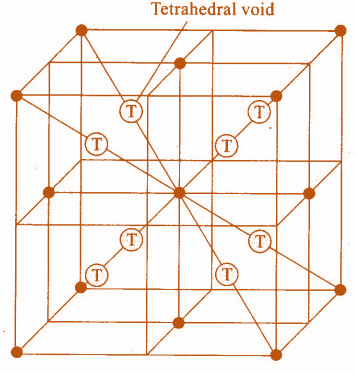
So, the correct answer is “Option D”.
Note: Face Centered Cubic (fcc) or Cubic Close Packed (ccp) are two dissimilar names for the same crystal lattice. In the maximum cubic crystal systems there is a presence of greater than one atom per one cubic unit cell.
Complete step by step answer:
-If there is an ‘n’ number of unit cells then the number of voids is double the number of unit cells means ‘2n’.
-The void in a cube has a smaller volume and the coordination number for the tetrahedral void is four.
-As cubic close packing is similar to face-centered cubic (fcc) then the number of atoms in a fcc structure is 4 each to one unit cell
-We know that the number of tetrahedral voids = 2n
-Thus the total number of tetrahedral voids = 2 (4) = 8.
-Therefore 8 tetrahedral voids are present within the unit cell.
- The representation of the 8 tetrahedral voids within the unit cells can see in the following structure.

So, the correct answer is “Option D”.
Note: Face Centered Cubic (fcc) or Cubic Close Packed (ccp) are two dissimilar names for the same crystal lattice. In the maximum cubic crystal systems there is a presence of greater than one atom per one cubic unit cell.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




