
In the given reaction, carbonyl compound (x) and Grignard reagent (y) can be.
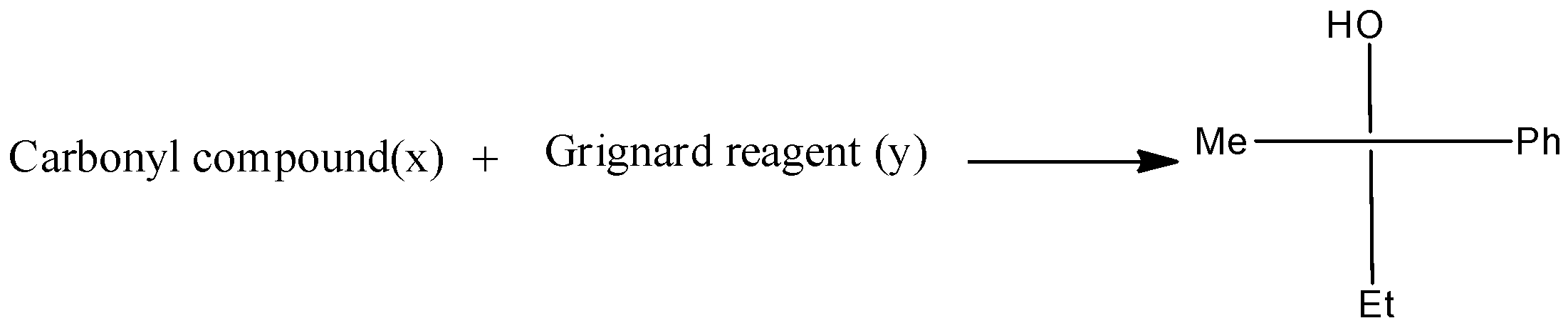
A)
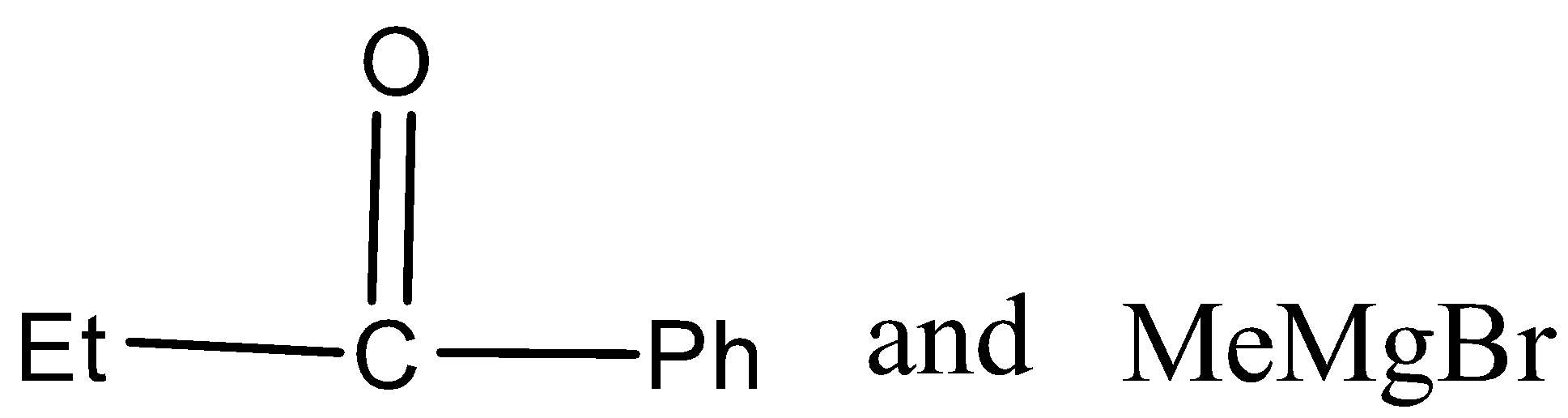
B)
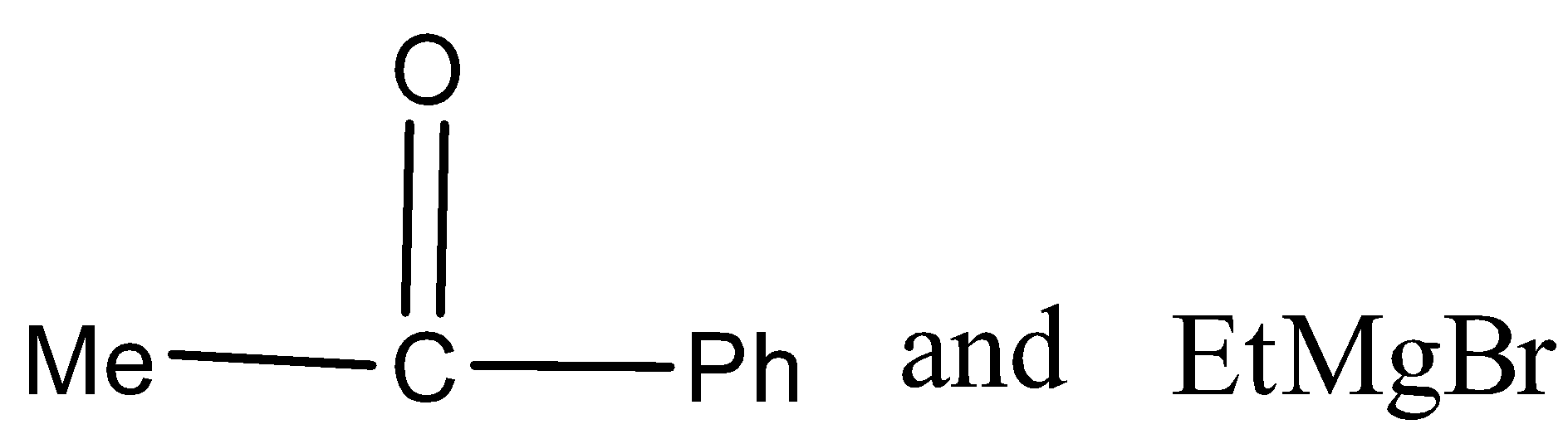
C)
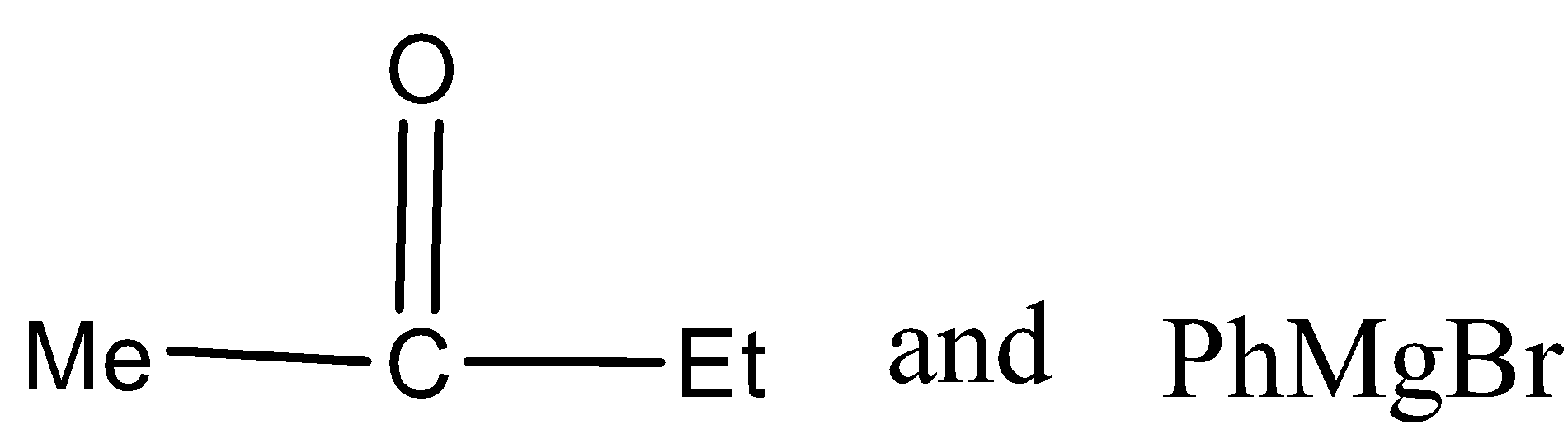
D)
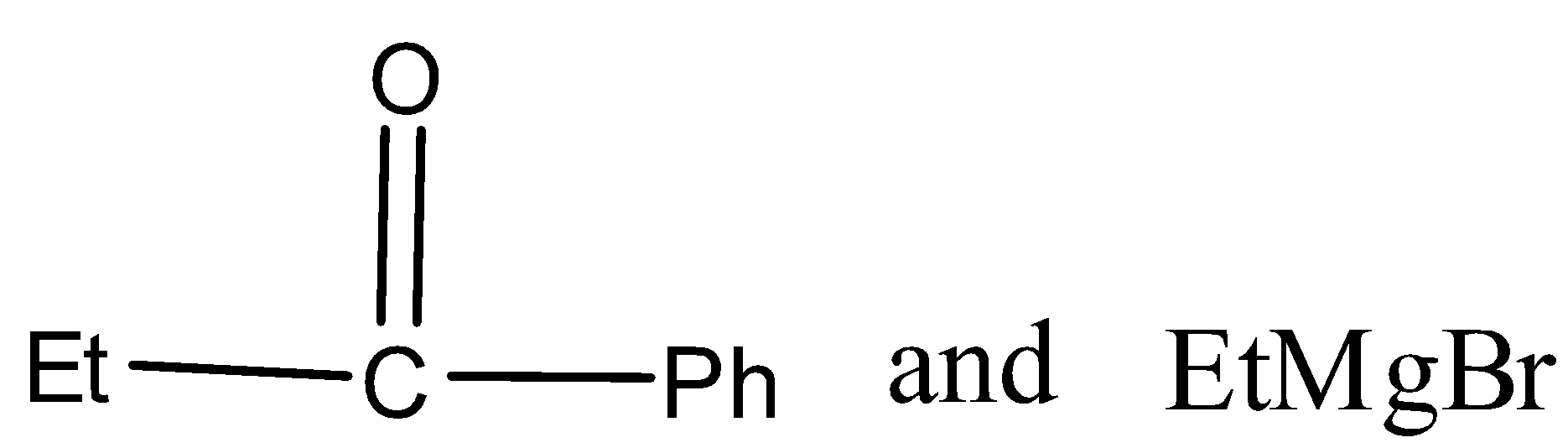
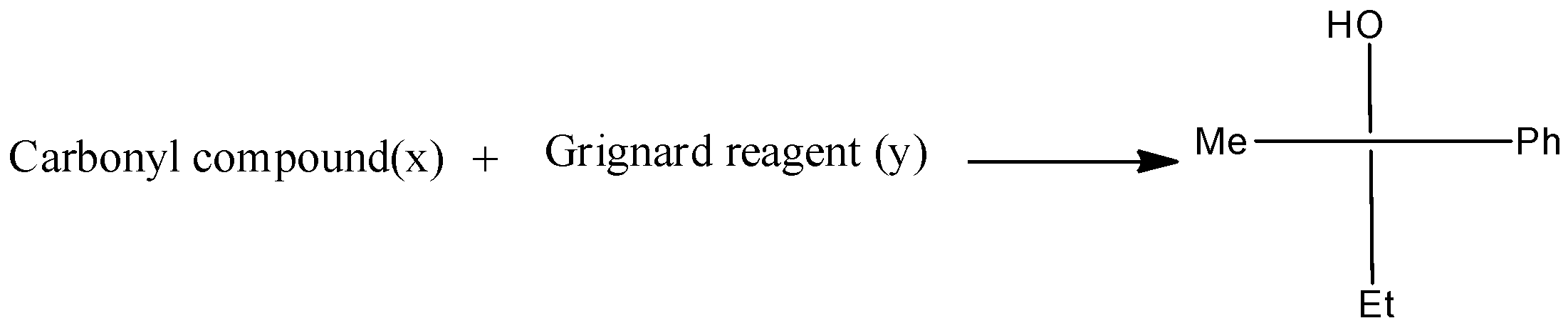
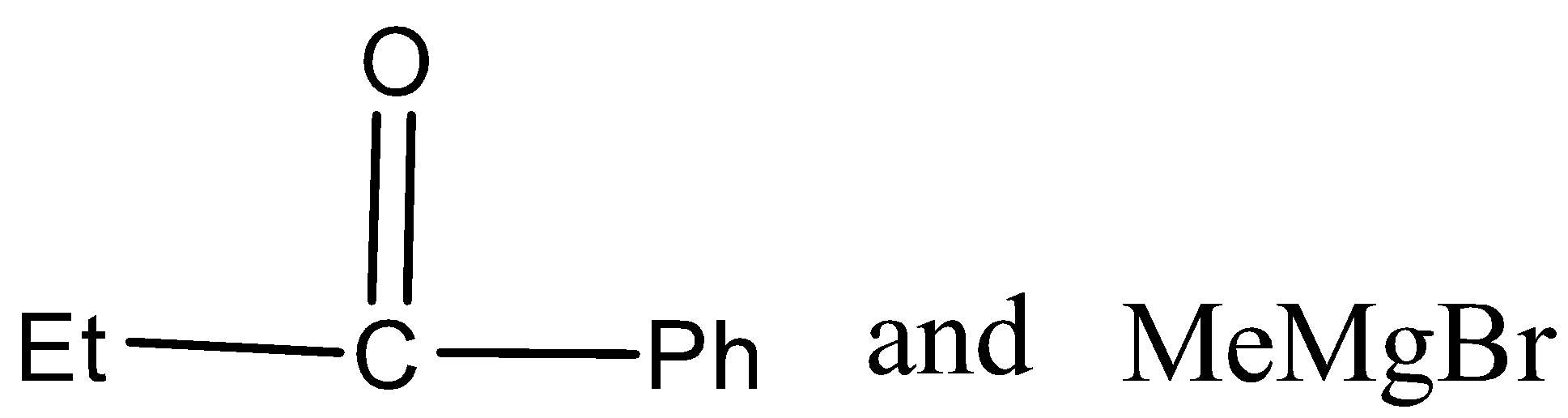
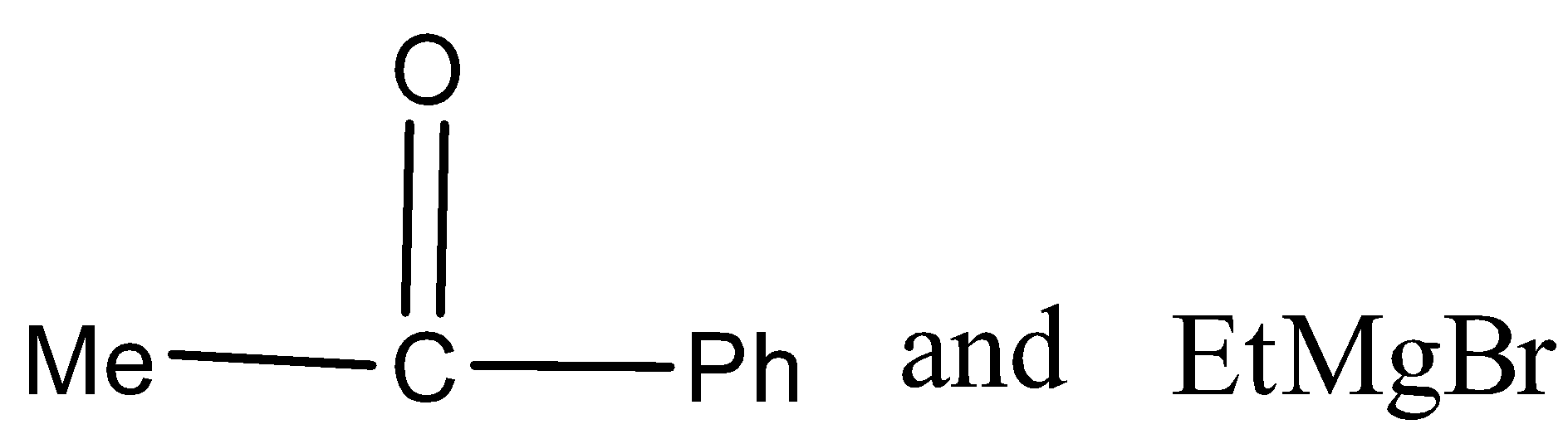
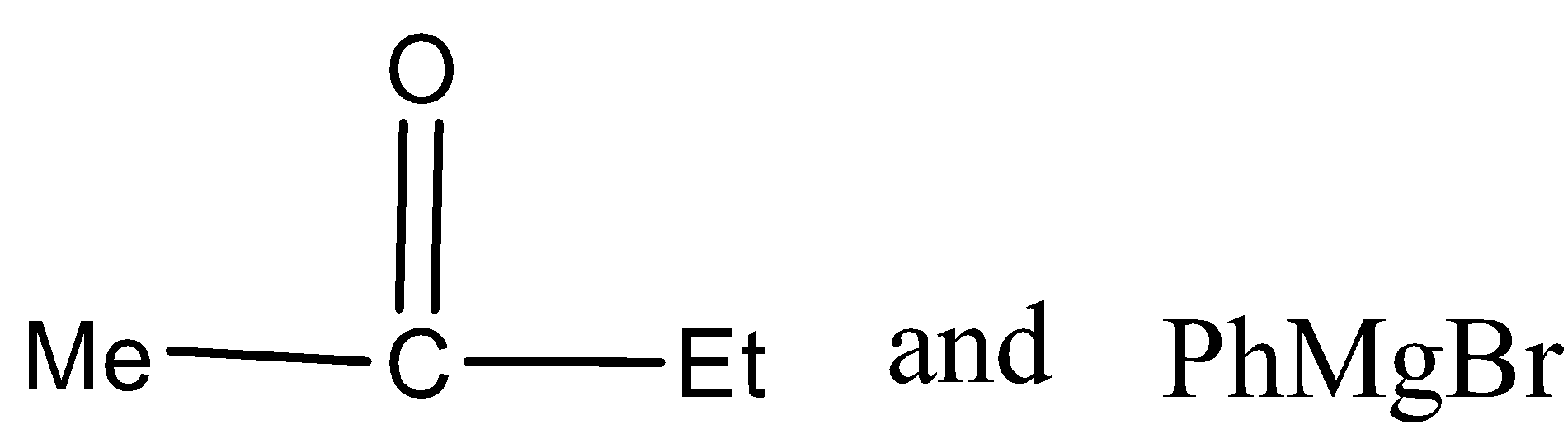
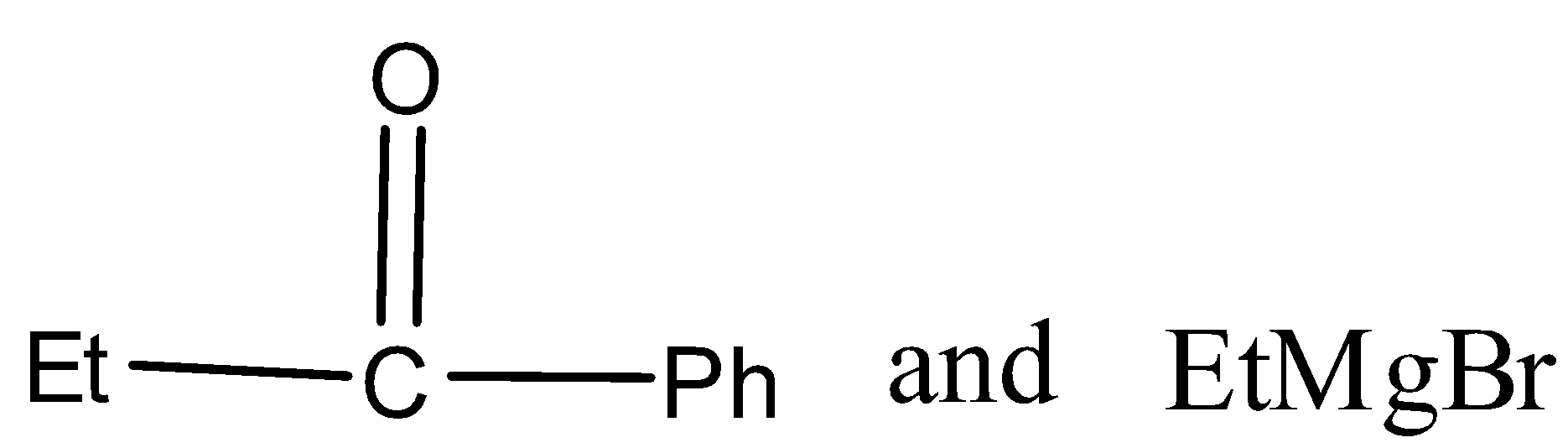
Answer
582.3k+ views
Hint:In the product there is a formation of a new carbon-carbon bond also we know that the magnesium in the Grignard reagent is electropositive in nature and which makes the alkyl group a partially negative compound. Thus, involving a nucleophilic substitution in the reaction.
Complete step by step answer:
First, we know what Grignard Reaction is,
The reaction in which the addition of an organo magnesium halide to a ketone or aldehyde, to give a tertiary or secondary alcohol is called Grignard reaction. The Grignard reagent reacts with formaldehyde results in the formation of primary alcohol. Grignard Reagents also are utilized in the subsequent important reactions: The addition of a more than a Grignard reagent to an ester or lactones gives a tertiary alcohol during which two alkyl groups are an equivalent, and therefore the addition of a Grignard reagent to a nitrile produces an unsymmetrical ketone via a metallic imine intermediate.
The given reaction is written as,
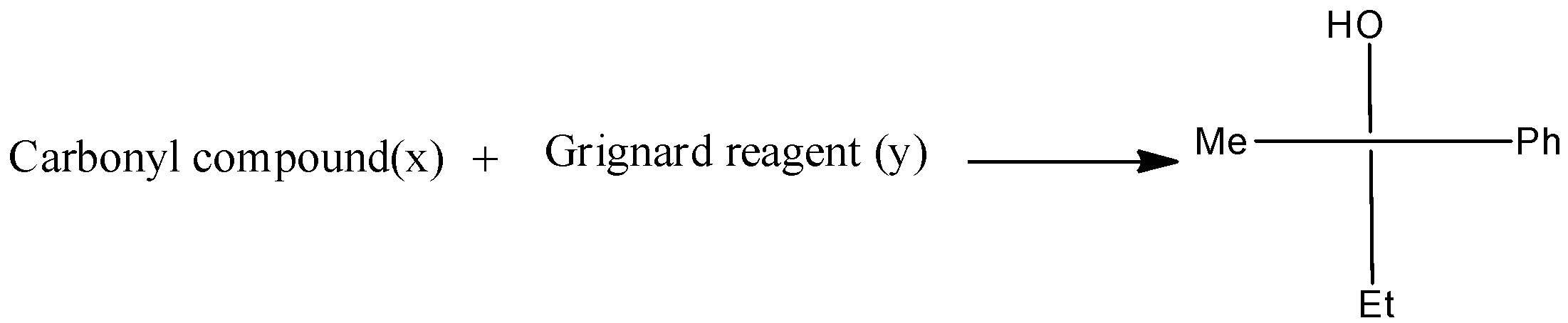
There are three different R groups in the product, thus three different carbonyl compounds and three different Grignard reagents are used to obtain the product.
Therefore, the option A, B, and C is correct.
Note:We must remember that the Grignard reagent is used to polarize the bond between carbon and magnesium and it makes the reagent to act as a good nucleophile and strong base. Avoid the reactions with water because it reacts with water and forms alkanes so in order to avoid the formation of alkane’s diethyl ether is used as a solvent.
Complete step by step answer:
First, we know what Grignard Reaction is,
The reaction in which the addition of an organo magnesium halide to a ketone or aldehyde, to give a tertiary or secondary alcohol is called Grignard reaction. The Grignard reagent reacts with formaldehyde results in the formation of primary alcohol. Grignard Reagents also are utilized in the subsequent important reactions: The addition of a more than a Grignard reagent to an ester or lactones gives a tertiary alcohol during which two alkyl groups are an equivalent, and therefore the addition of a Grignard reagent to a nitrile produces an unsymmetrical ketone via a metallic imine intermediate.
The given reaction is written as,
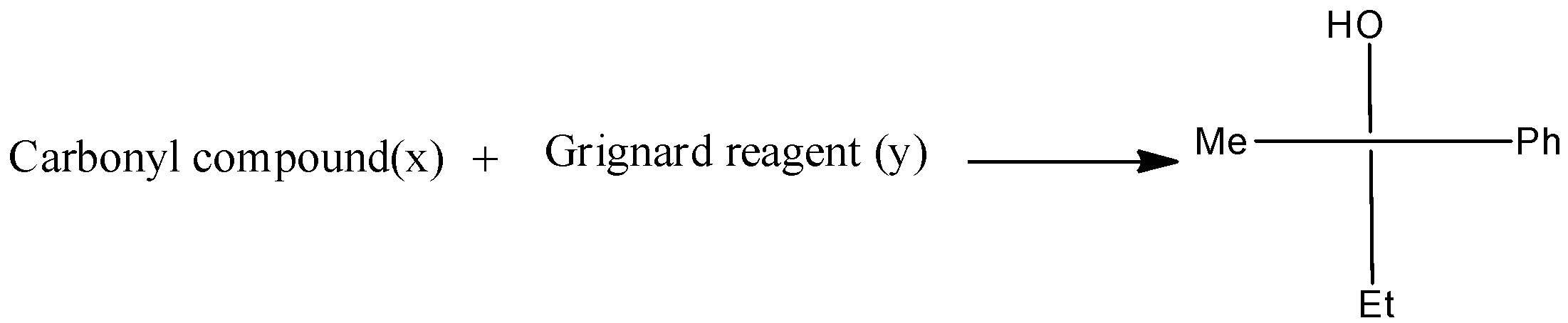
There are three different R groups in the product, thus three different carbonyl compounds and three different Grignard reagents are used to obtain the product.
Therefore, the option A, B, and C is correct.
Note:We must remember that the Grignard reagent is used to polarize the bond between carbon and magnesium and it makes the reagent to act as a good nucleophile and strong base. Avoid the reactions with water because it reacts with water and forms alkanes so in order to avoid the formation of alkane’s diethyl ether is used as a solvent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




