
In the Hofmann mustard oil reaction of primary amines, the black precipitate is due to -
(A) HgS
(B) $A{{g}_{2}}S$
(C) CuS
(D) BaS
Answer
523.1k+ views
Hint: The black precipitate is formed due to a compound that is virtually insoluble in water. The compound is dimorphic with 2 crystal forms – cinnabar and metacinnabar. It is also called vermillion when it is used as a pigment.
Complete-step- by- step answer:
The Hofmann mustard oil reaction is an organic reaction in which primary amines are warmed with alcoholic carbon disulphide ($C{{S}_{2}}$ ) and then heated with excess of mercuric chloride ($HgC{{l}_{2}}$ ) leading to the formation of isothiocyanates. These isothiocyanates are responsible for the pungent smell that arises in the reaction. This smell is similar to that of mustard oil.
The Hofmann mustard oil reaction is used as a test to distinguish between primary, secondary and tertiary amines.
Let us now see how each of the amines react to the Hoffman mustard oil test.
i) Tertiary amines do not react with any of the reagents ($C{{S}_{2}}$ and $HgC{{l}_{2}}$).
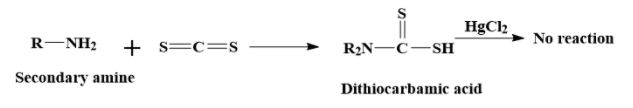
ii) Secondary amines react with carbon disulphide to form dithiocarbamic acids. But in the next step, it does not react with the mercuric chloride.
 iii) Primary amines when treated with carbon disulphide, followed by heating with excess $HgC{{l}_{2}}$ gives isothiocyanates, that are responsible for the pungent smell that is similar to that of mustard oil.
iii) Primary amines when treated with carbon disulphide, followed by heating with excess $HgC{{l}_{2}}$ gives isothiocyanates, that are responsible for the pungent smell that is similar to that of mustard oil.
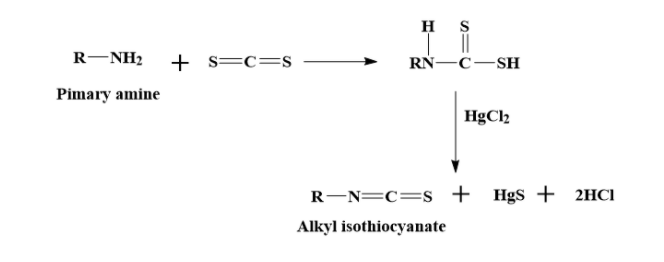
During the reaction, we observe that a black insoluble precipitate is formed. This insoluble precipitate is actually HgS that is formed during the reaction.
Therefore, the correct answer is (A).
Note: This is the test that is used to distinguish between primary, secondary and tertiary amines. It is important to remember that aromatic primary amines also give this test. Hence, this test cannot be used to distinguish between aromatic and aliphatic amines.
Complete-step- by- step answer:
The Hofmann mustard oil reaction is an organic reaction in which primary amines are warmed with alcoholic carbon disulphide ($C{{S}_{2}}$ ) and then heated with excess of mercuric chloride ($HgC{{l}_{2}}$ ) leading to the formation of isothiocyanates. These isothiocyanates are responsible for the pungent smell that arises in the reaction. This smell is similar to that of mustard oil.
The Hofmann mustard oil reaction is used as a test to distinguish between primary, secondary and tertiary amines.
Let us now see how each of the amines react to the Hoffman mustard oil test.
i) Tertiary amines do not react with any of the reagents ($C{{S}_{2}}$ and $HgC{{l}_{2}}$).
ii) Secondary amines react with carbon disulphide to form dithiocarbamic acids. But in the next step, it does not react with the mercuric chloride.


During the reaction, we observe that a black insoluble precipitate is formed. This insoluble precipitate is actually HgS that is formed during the reaction.
Therefore, the correct answer is (A).
Note: This is the test that is used to distinguish between primary, secondary and tertiary amines. It is important to remember that aromatic primary amines also give this test. Hence, this test cannot be used to distinguish between aromatic and aliphatic amines.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




