
In the structure of \[I{{F}_{7}}\] (Iodine heptafluoride) ----------.
(A) \[{{d}_{xy}},{{d}_{x}},{{d}_{z}}\] orbitals are involved in hybridization
(B) Axial bonds are longer than equatorial bonds
(C) There are 10 different orthogonal angels
(D)The resultant geometry is a decahedral
Answer
589.8k+ views
Hint: Iodine heptafluoride, also called iodine (VII) fluoride. It is an interhalogen compound having a chemical formula of\[I{{F}_{7}}\]. As per VSEPR theory the structure of \[I{{F}_{7}}\] is pentagonal bipyramidal with a hybridization of \[s{{p}^{3}}{{d}^{3}}\].
Complete step by step solution:
The structure of \[I{{F}_{7}}\]is as follows.
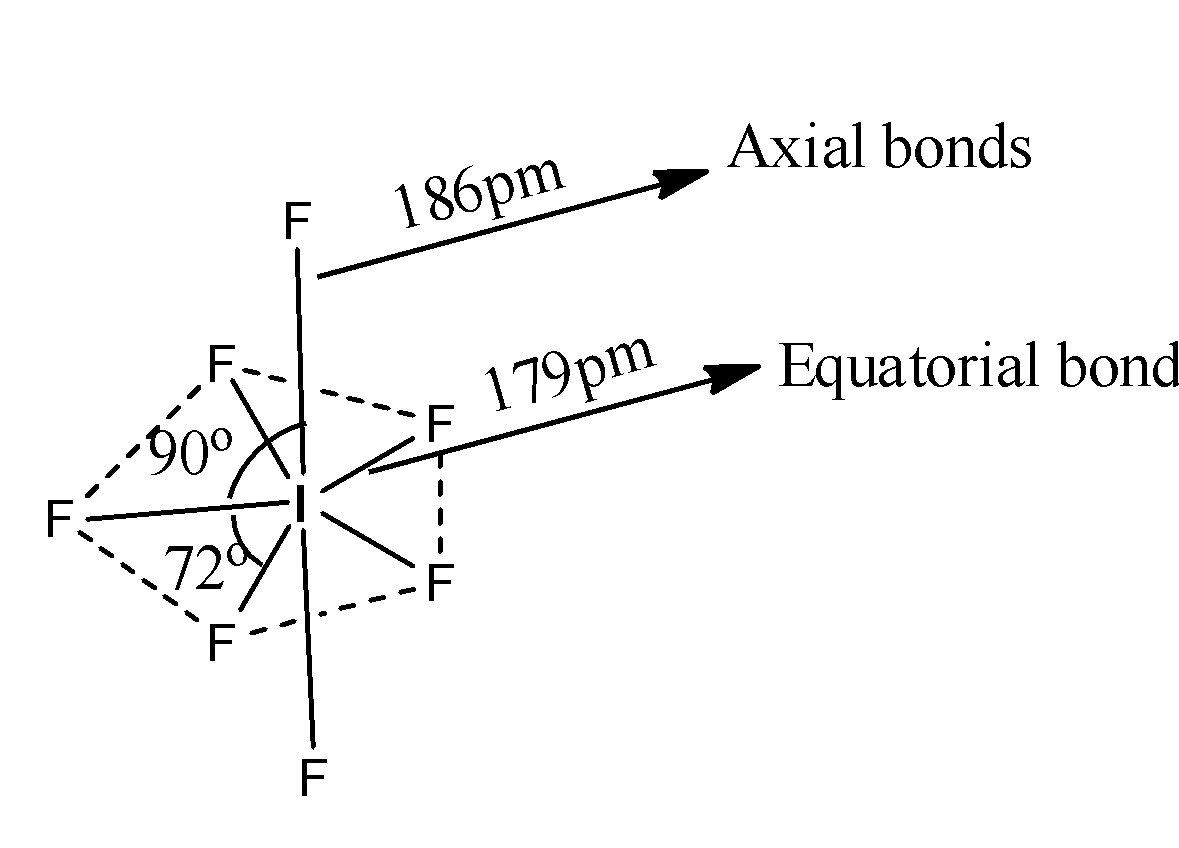
The hybridization of Iodine in \[I{{F}_{7}}\] is \[s{{p}^{3}}{{d}^{3}}\] and the overlapping of fluorine with iodine orbitals we can see as follows.
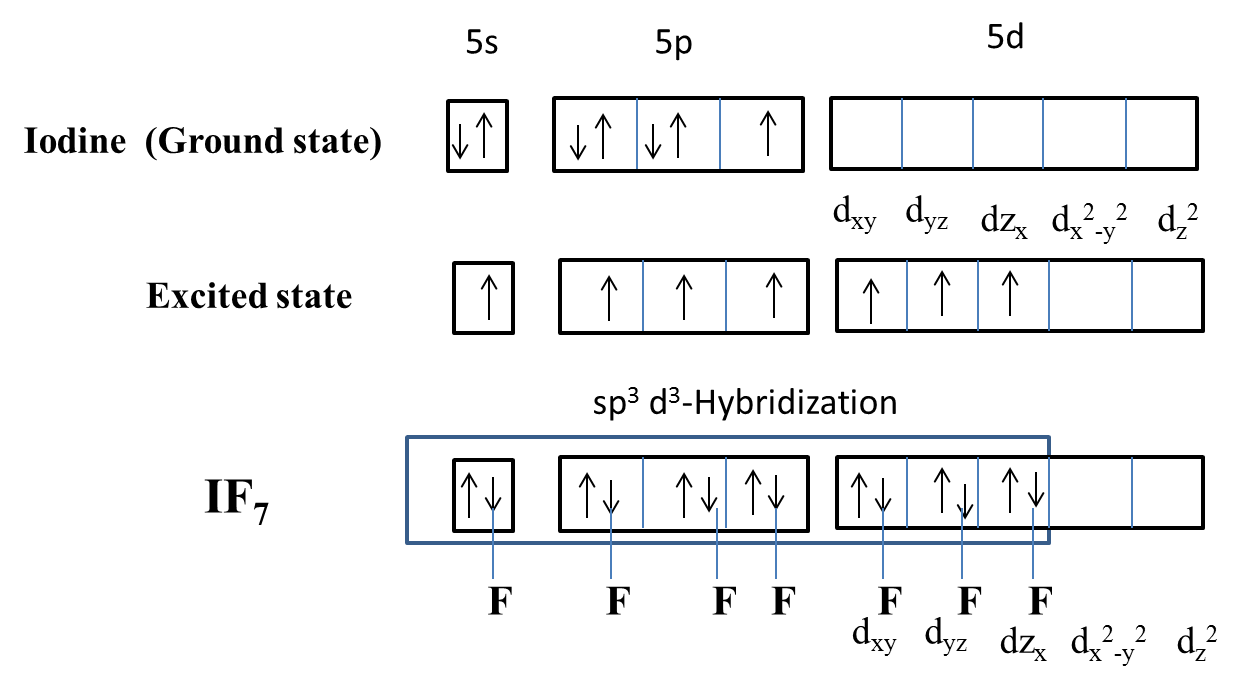
Now coming to the given options, option A, \[{{d}_{xy}},{{d}_{x}},{{d}_{z}}\] orbitals are involved in hybridization, it is wrong because in the hybridization the orbitals involved are \[{{d}_{xy}},{{d}_{yz}},{{d}_{zx}}\] not \[{{d}_{xy}},{{d}_{x}},{{d}_{z}}\]. So, option A is wrong.
Coming to option B, Axial bonds are longer than equatorial bonds, yes it is true. Because from the structure we can say that the length of the axial bonds are 186pm, and length of the equatorial bonds are 179pm. So, axial bonds are longer than equatorial bonds. So, option B is correct.
Coming to option C, There are 10 different orthogonal angels. No there are only two types of orthogonal angles, there are 90 and 72. So, option C is wrong.
Coming to option D, The resultant geometry is decahedral, it is wrong. Because the resultant geometry is pentagonal bipyramidal. So, option D is wrong.
So, the correct option is B.
Note: The structure of the\[I{{F}_{5}}\]is a square pyramid or distorted octahedral and the structure of the\[I{{F}_{7}}\] is pentagonal bipyramidal. Both structures are different.
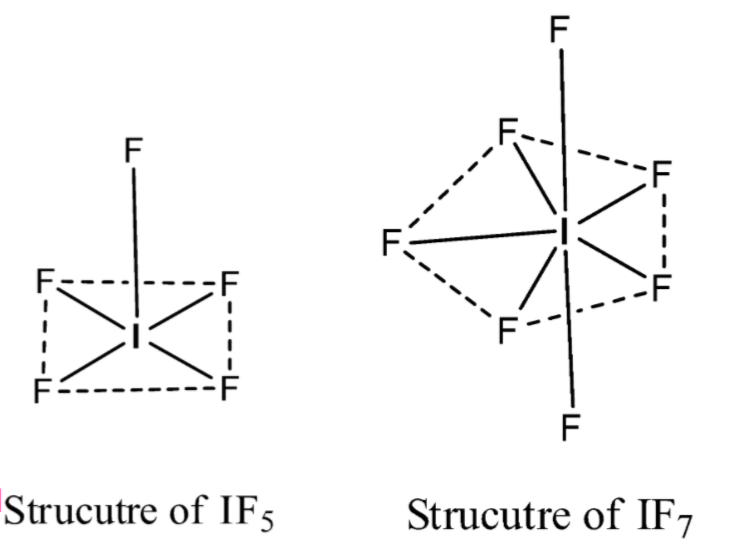
The hybridization of Iodine in \[I{{F}_{5}}\]is\[s{{p}^{3}}{{d}^{2}}\]
The hybridization of Iodine in \[I{{F}_{7}}\]is\[s{{p}^{3}}{{d}^{3}}\]
Complete step by step solution:
The structure of \[I{{F}_{7}}\]is as follows.

The hybridization of Iodine in \[I{{F}_{7}}\] is \[s{{p}^{3}}{{d}^{3}}\] and the overlapping of fluorine with iodine orbitals we can see as follows.

Now coming to the given options, option A, \[{{d}_{xy}},{{d}_{x}},{{d}_{z}}\] orbitals are involved in hybridization, it is wrong because in the hybridization the orbitals involved are \[{{d}_{xy}},{{d}_{yz}},{{d}_{zx}}\] not \[{{d}_{xy}},{{d}_{x}},{{d}_{z}}\]. So, option A is wrong.
Coming to option B, Axial bonds are longer than equatorial bonds, yes it is true. Because from the structure we can say that the length of the axial bonds are 186pm, and length of the equatorial bonds are 179pm. So, axial bonds are longer than equatorial bonds. So, option B is correct.
Coming to option C, There are 10 different orthogonal angels. No there are only two types of orthogonal angles, there are 90 and 72. So, option C is wrong.
Coming to option D, The resultant geometry is decahedral, it is wrong. Because the resultant geometry is pentagonal bipyramidal. So, option D is wrong.
So, the correct option is B.
Note: The structure of the\[I{{F}_{5}}\]is a square pyramid or distorted octahedral and the structure of the\[I{{F}_{7}}\] is pentagonal bipyramidal. Both structures are different.

The hybridization of Iodine in \[I{{F}_{5}}\]is\[s{{p}^{3}}{{d}^{2}}\]
The hybridization of Iodine in \[I{{F}_{7}}\]is\[s{{p}^{3}}{{d}^{3}}\]
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




