
In this reaction find out what is P.

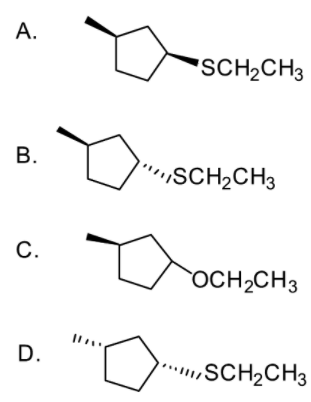


Answer
561k+ views
Hint: The purpose of making alcohol react with tosyl chloride is to convert the poor leaving group –OH into good leaving group –OTs , so later on nucleophilic substitution can be performed easily that was initially difficult with –OH group. Also as this is a secondary alcohol and pyridine is polar aprotic solvent so reaction will take place according to ${{S}_{N}}2$ mechanism.
Complete step by step answer:
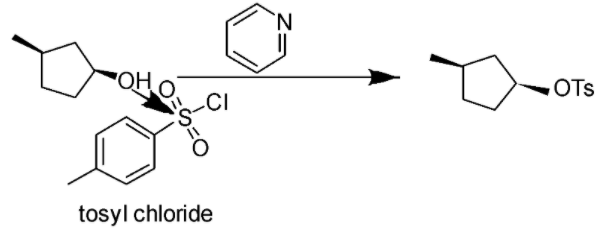
When tosyl chloride will react with given alcohol then oxygen will donate its lone pair to the vacant orbital of sulfur and create a bond, chlorine will leave the group and take the hydrogen attached to oxygen along with it as oxygen-hydrogen bond got weaker because of the electron density transfer to the sulfur atom. The HCl formed will be neutralized by pyridine.
Reaction is shown below:
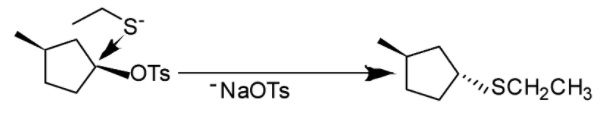
Now this Tosylate is made to react with sodium ethane thiolate. Now here reaction will take place according to ${{S}_{N}}2$ mechanism and inversion of configuration is going to take place. As ethane thiolate ions will attack from the back side and displace the good leaving group –OTs.
Reaction is shown below:
- So product P formed will be
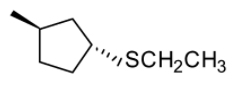
The correct answer is option “B” .
Additional Information : If reaction of alcohol was taking place with thionyl chloride in place of tosyl chloride, the corresponding chloride would have formed with inversion of configuration.
Note: In case of secondary alcohols and secondary alkyl halides while performing nucleophilic substitution keep an eye on solvent. As in this case, the solvent will decide the mechanism. If solvent is polar protic reaction will take place via ${{S}_{N}}1$ mechanism and in case of polar aprotic solvent reaction will take place via ${{S}_{N}}2$ mechanism.
Complete step by step answer:
When tosyl chloride will react with given alcohol then oxygen will donate its lone pair to the vacant orbital of sulfur and create a bond, chlorine will leave the group and take the hydrogen attached to oxygen along with it as oxygen-hydrogen bond got weaker because of the electron density transfer to the sulfur atom. The HCl formed will be neutralized by pyridine.
Reaction is shown below:

Now this Tosylate is made to react with sodium ethane thiolate. Now here reaction will take place according to ${{S}_{N}}2$ mechanism and inversion of configuration is going to take place. As ethane thiolate ions will attack from the back side and displace the good leaving group –OTs.
Reaction is shown below:

- So product P formed will be

The correct answer is option “B” .
Additional Information : If reaction of alcohol was taking place with thionyl chloride in place of tosyl chloride, the corresponding chloride would have formed with inversion of configuration.
Note: In case of secondary alcohols and secondary alkyl halides while performing nucleophilic substitution keep an eye on solvent. As in this case, the solvent will decide the mechanism. If solvent is polar protic reaction will take place via ${{S}_{N}}1$ mechanism and in case of polar aprotic solvent reaction will take place via ${{S}_{N}}2$ mechanism.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




