
In which of the following alkene, a hydride shift occur upon addition of $HCl$
A.
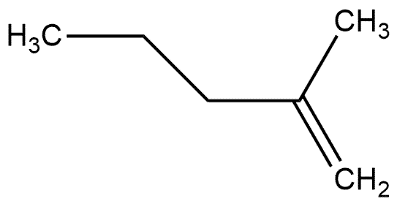
B.
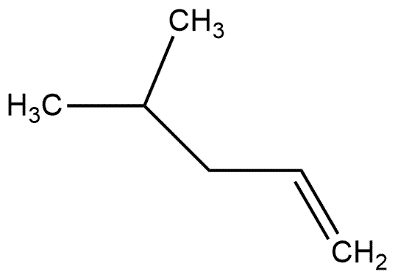
C.
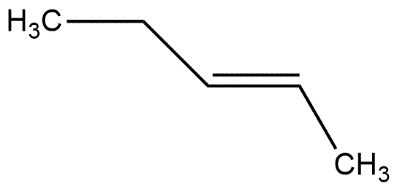
D.
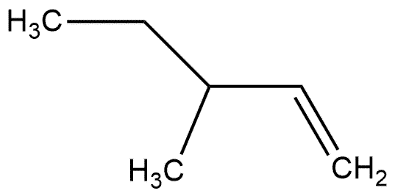




Answer
565.2k+ views
Hint: In organic chemistry carbocation rearrangements are very much common. These rearrangements can be defined as the movement of a carbocation from an unstable state to a more stable state by going through a number of structural organizational shifts within the molecule.
Complete Solution :
Now to find out which of the given compounds will show hydride shift we first have to discuss hydride shift.
- Hydride shift is the movement of a hydrogen atom from one carbon to an adjacent charged carbon atom of the same compound. Most frequently the carbocation rearrangements occur in secondary carbocations. In most of the cases we generally see two products called a major product and a minor product. The major product is basically the rearranged product that is more stable or we can say more substituted while the minor product is basically less stable or less substituted. If a secondary carbocation is vicinal to a tertiary carbon atom bearing a hydrogen atom then a hydride shift occurs. We can also call it a 1,2-hydride shift. This shift is possible when there is a positive charge on the carbon atom where its adjacent carbon atom has a removable hydrogen atom.
- Now we know that tertiary carbocation is more stable as compared to primary and secondary carbocation so the alkene given in option D form tertiary carbocation when hydrogen shift occurs upon the addition of $HCl$.
So, the correct answer is “Option D”.
Note: During carbocation rearrangement once the carbocation has shifted over to a different carbon we can say that there is a structural isomer of the initial molecule. While alkene are those compounds which double bond present in them and these compounds are ended with the suffix ene.
Complete Solution :
Now to find out which of the given compounds will show hydride shift we first have to discuss hydride shift.
- Hydride shift is the movement of a hydrogen atom from one carbon to an adjacent charged carbon atom of the same compound. Most frequently the carbocation rearrangements occur in secondary carbocations. In most of the cases we generally see two products called a major product and a minor product. The major product is basically the rearranged product that is more stable or we can say more substituted while the minor product is basically less stable or less substituted. If a secondary carbocation is vicinal to a tertiary carbon atom bearing a hydrogen atom then a hydride shift occurs. We can also call it a 1,2-hydride shift. This shift is possible when there is a positive charge on the carbon atom where its adjacent carbon atom has a removable hydrogen atom.
- Now we know that tertiary carbocation is more stable as compared to primary and secondary carbocation so the alkene given in option D form tertiary carbocation when hydrogen shift occurs upon the addition of $HCl$.
So, the correct answer is “Option D”.
Note: During carbocation rearrangement once the carbocation has shifted over to a different carbon we can say that there is a structural isomer of the initial molecule. While alkene are those compounds which double bond present in them and these compounds are ended with the suffix ene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




