
Indium impurity in germanium makes –
$\left( {\text{A}} \right){\text{ }}$ n-type
$\left( {\text{B}} \right){\text{ }}$ p-type
$\left( {\text{C}} \right){\text{ }}$ Insulator
$\left( {\text{D}} \right){\text{ }}$ Intrinsic
Answer
548.1k+ views
Hint:
1. Indium is an element of the \[{\text{13th}}\] group (having atomic number $49$ ) in the periodic table. and it contains three electrons in the outer orbit $(2,8,18,18,3)$. That’s why it is called a trivalent compound.
2. Note that, the germanium has four electrons in the outer orbit. The hole is created due to an incomplete bond of the indium.
Complete step by step answer:
When a small amount of trivalent impurity is added to pure Germanium or silicon atom crystal, then a p-type semiconductor is formed. The addition of trivalent impurity creates a large no. of holes in the host crystal element. To elaborate on the formation of p-type semiconductor, we will introduce a trivalent element impurity into the lattice of a pure silicon crystal. The trivalent atom has \[{\text{3}}\] valence electrons and form covalent bonds with neighbouring atoms. The 4th bond is incomplete in the atom. After that, the trivalent atom attracts an electron from an adjacent atom thereby completing the 4th bond and will form a hole in the adjacent atom. Indium is trivalent, on doping with it, the intrinsic semiconductor becomes P-type.
Hence, Indium impurity in germanium makes p-type semiconductor.
$\therefore $The correct option is $\left( {\text{B}} \right)$.
Additional information:
The absence of an electron in a particular place in an atom is called a hole. Hole is an electric charge carrier that has a positive charge.
We know that a trivalent impurity atom provides \[1\] positive charged hole, it results in an enormous increase in the number of holes. The impure crystals obtained by this process is called P-type semiconductor where P denotes the positive charge on the hole. Thus, the majority charge carrier in a P-type semiconductor is holes. The trivalent impurity atoms are referred to as acceptors because each atom accepts an electron when it is introduced into the host crystal.
Note:
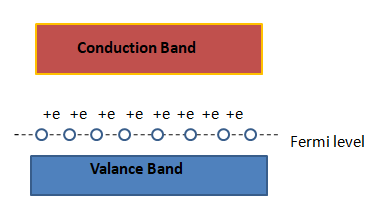
If we notice the energy band of a p-type semiconductor , the fermi level can be seen. The fermi level keeps going towards the valence band more when the doping increases. Then the excited electrons can easily be brought to the fermi level. That's why there hole is created in the valence band due to lack of electrons. Note that the motion of holes are not the same as the electrons and that's why it can be said that the effective mass of a hole is greater than that of an electron.
1. Indium is an element of the \[{\text{13th}}\] group (having atomic number $49$ ) in the periodic table. and it contains three electrons in the outer orbit $(2,8,18,18,3)$. That’s why it is called a trivalent compound.
2. Note that, the germanium has four electrons in the outer orbit. The hole is created due to an incomplete bond of the indium.
Complete step by step answer:
When a small amount of trivalent impurity is added to pure Germanium or silicon atom crystal, then a p-type semiconductor is formed. The addition of trivalent impurity creates a large no. of holes in the host crystal element. To elaborate on the formation of p-type semiconductor, we will introduce a trivalent element impurity into the lattice of a pure silicon crystal. The trivalent atom has \[{\text{3}}\] valence electrons and form covalent bonds with neighbouring atoms. The 4th bond is incomplete in the atom. After that, the trivalent atom attracts an electron from an adjacent atom thereby completing the 4th bond and will form a hole in the adjacent atom. Indium is trivalent, on doping with it, the intrinsic semiconductor becomes P-type.
Hence, Indium impurity in germanium makes p-type semiconductor.
$\therefore $The correct option is $\left( {\text{B}} \right)$.
Additional information:
The absence of an electron in a particular place in an atom is called a hole. Hole is an electric charge carrier that has a positive charge.
We know that a trivalent impurity atom provides \[1\] positive charged hole, it results in an enormous increase in the number of holes. The impure crystals obtained by this process is called P-type semiconductor where P denotes the positive charge on the hole. Thus, the majority charge carrier in a P-type semiconductor is holes. The trivalent impurity atoms are referred to as acceptors because each atom accepts an electron when it is introduced into the host crystal.
Note:

If we notice the energy band of a p-type semiconductor , the fermi level can be seen. The fermi level keeps going towards the valence band more when the doping increases. Then the excited electrons can easily be brought to the fermi level. That's why there hole is created in the valence band due to lack of electrons. Note that the motion of holes are not the same as the electrons and that's why it can be said that the effective mass of a hole is greater than that of an electron.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




