
Inorganic graphite is
(a) \[{{B}_{2}}{{H}_{6}}\]
(b) \[BN\]
(c) \[B{{F}_{3}}\]
(d) \[{{B}_{2}}{{N}_{3}}{{H}_{6}}\]
Answer
233.1k+ views
Hint: Graphite is an allotrope of carbon. It has layered structures and has carbon atoms in \[s{{p}^{2}}\] hybridised state. The inorganic graphite is a molecule similar to graphite but is inorganic.
Complete step by step solution:
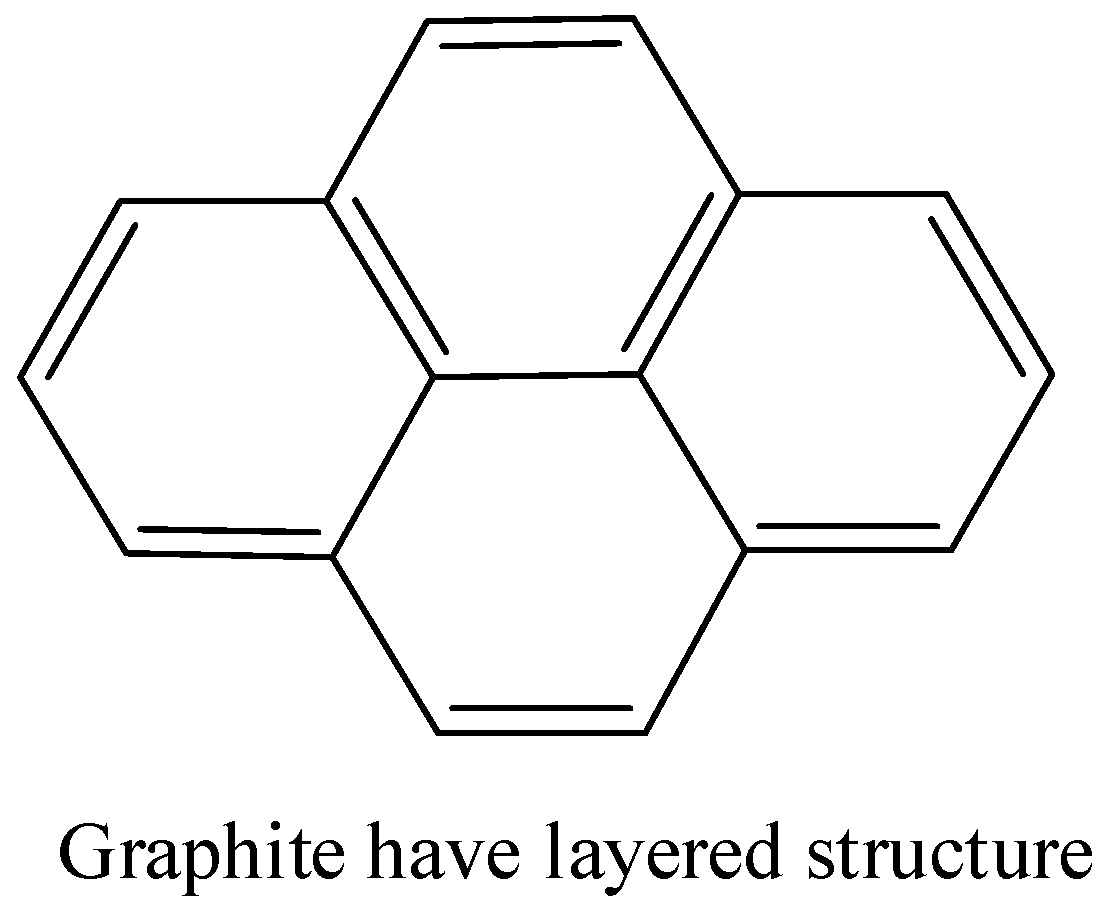
- Graphite is a crystalline form of carbon in which carbon is arranged in hexagonal structure. There are layers of \[s{{p}^{2}}\] hybridised carbon and the layers are held together by weak van der waal forces.
- Inorganic graphite means it is an inorganic compound having similar structure to graphite. Let us compare this with each option given in the question.
- Diborane has four hydrogen atoms and two boron atoms in the same plane. Each boron atom is \[s{{p}^{3}}\] hybridised and has four hybrid orbitals. Does not have any layered structure. So, it is not considered as inorganic graphite.
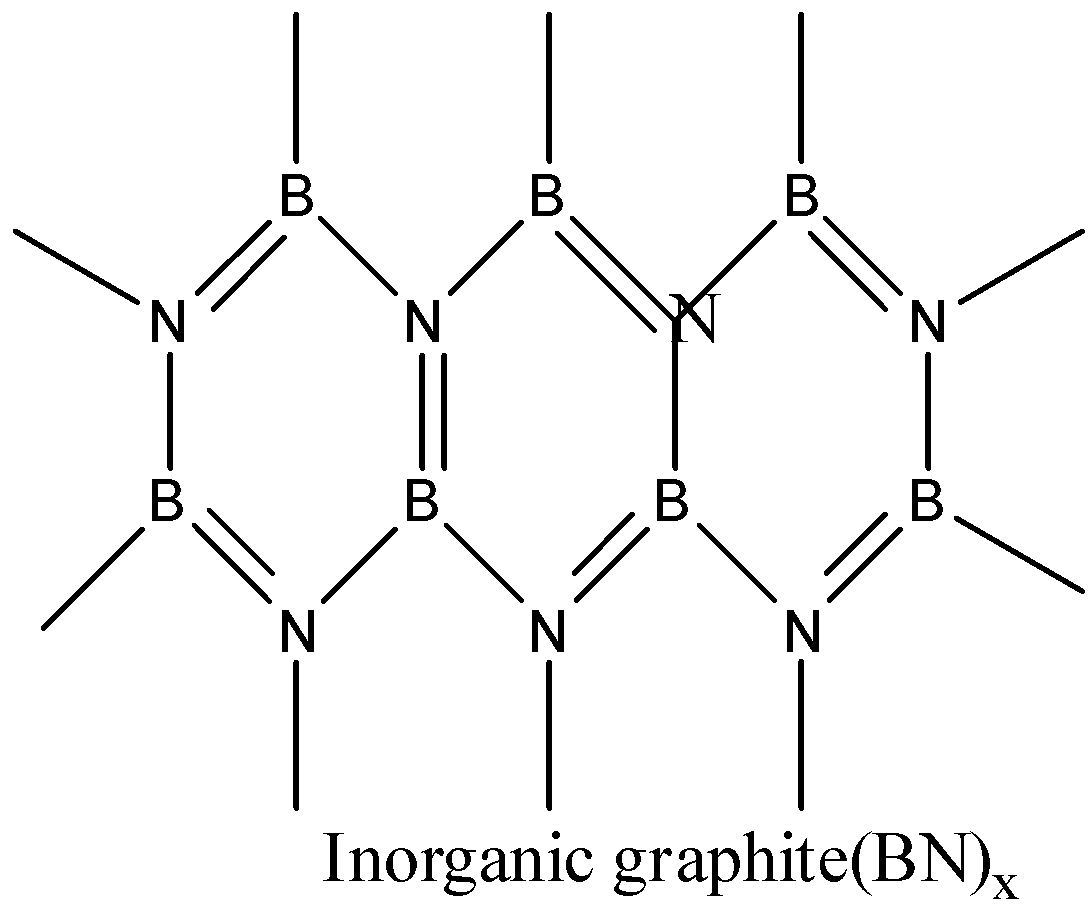
- Boron nitride is a compound consisting of boron and nitrogen. There are many structures of BN. It is found in amorphous form, hexagonal form, cubic form and wurtzite form. The hexagonal boron nitride is having a layered structure which is similar to that of graphite. These layers are also held together by weak van der waal forces. But it differs a little bit with graphite layers because the atoms are eclipsed, that is boron atoms are lying above nitrogen atoms. So, BN has some similarities with graphite.
- Boron trifluoride has one boron and 3 fluorine. It has a trigonal planar structure.
- Borazine is a six member ring, with two borons, three nitrogen and 6 hydrogen. It has a structure similar to benzene.
Thus, boron nitride BN is known as inorganic graphite. The correct answer is option (b).
Note: Inorganic graphite can be confused with borazine. But borazine is known as inorganic benzene, it has similarities with the structure of benzene.
Complete step by step solution:
- Graphite is a crystalline form of carbon in which carbon is arranged in hexagonal structure. There are layers of \[s{{p}^{2}}\] hybridised carbon and the layers are held together by weak van der waal forces.
- Inorganic graphite means it is an inorganic compound having similar structure to graphite. Let us compare this with each option given in the question.
- Diborane has four hydrogen atoms and two boron atoms in the same plane. Each boron atom is \[s{{p}^{3}}\] hybridised and has four hybrid orbitals. Does not have any layered structure. So, it is not considered as inorganic graphite.
- Boron nitride is a compound consisting of boron and nitrogen. There are many structures of BN. It is found in amorphous form, hexagonal form, cubic form and wurtzite form. The hexagonal boron nitride is having a layered structure which is similar to that of graphite. These layers are also held together by weak van der waal forces. But it differs a little bit with graphite layers because the atoms are eclipsed, that is boron atoms are lying above nitrogen atoms. So, BN has some similarities with graphite.
- Boron trifluoride has one boron and 3 fluorine. It has a trigonal planar structure.
- Borazine is a six member ring, with two borons, three nitrogen and 6 hydrogen. It has a structure similar to benzene.
Thus, boron nitride BN is known as inorganic graphite. The correct answer is option (b).


Note: Inorganic graphite can be confused with borazine. But borazine is known as inorganic benzene, it has similarities with the structure of benzene.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




