
Interconversion of the terminal to internal alkyne and vice versa takes place by the following reagents (A) and (B):
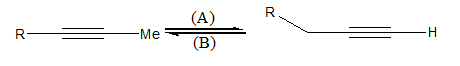
A.\[{\text{NaN}}{{\text{H}}_{\text{2}}}\] and alc. \[{\text{KOH}}\]
B. alc. \[{\text{KOH}}\] and \[{\text{NaN}}{{\text{H}}_{\text{2}}}\]
C. alc. \[{\text{KOH}}\] and P−2 catalyst
D.\[{\text{NaN}}{{\text{H}}_{\text{2}}}\]and Lindlar's catalyst
Answer
581.1k+ views
Hint: To answer this question recalls the methods for the preparation of alkyne from dihalides. Sodium amide is a strong reducing agent which removes the halide atoms and generates an alkyne.
Complete step by step answer:
\[{\text{NaN}}{{\text{H}}_{\text{2}}}\]is a strong base and also it can be an excellent nucleophile It’s used for deprotonation of weak acids and also for elimination reactions. As a strong base \[{\text{NaN}}{{\text{H}}_{\text{2}}}\] will deprotonate alkynes, alcohols, and a host of other functional groups with acidic protons such as esters and ketones. As a base, it’s often used in situations where a strong, small base is required.
Thus, \[{\text{NaN}}{{\text{H}}_{\text{2}}}\] converts internal alkyne to terminal alkyne and alcoholic \[{\text{KOH}}\] converts terminal alkyne to internal alkyne.
Hence, the correct option is A.
Additional information:
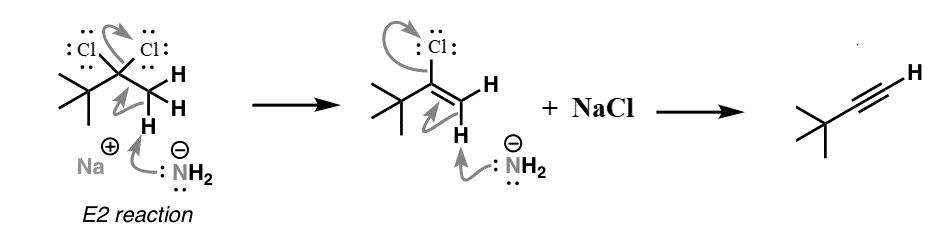
We know that Sodium amide (\[{\text{NaN}}{{\text{H}}_{\text{2}}}\]) is a strong base and is used for deprotonation of weak acids and also for elimination reactions. Treatment of either geminal dihalide (two halogens on one carbon) or vicinal dihalides (halogens on adjacent carbons) with two equivalents of \[{\text{NaN}}{{\text{H}}_{\text{2}}}\] results in the formation of alkynes. The mechanism of this reaction can be shown as:
Note:
We should keep in mind the formation of terminal alkynes by use of this reaction mechanism. The acidity of terminal alkynes plays an important role in major product determination when dihalides undergo base induced elimination reactions. High electronegativity of the triple bond in terminal alkynes makes the molecule acidic. Therefore, one of the base molecules will pull off the terminal hydrogen instead of one of the halides like we desire to happen in this reaction. This implies that we would need three bases for every terminal haloalkane instead of two to obtain an alkyne.
Complete step by step answer:
\[{\text{NaN}}{{\text{H}}_{\text{2}}}\]is a strong base and also it can be an excellent nucleophile It’s used for deprotonation of weak acids and also for elimination reactions. As a strong base \[{\text{NaN}}{{\text{H}}_{\text{2}}}\] will deprotonate alkynes, alcohols, and a host of other functional groups with acidic protons such as esters and ketones. As a base, it’s often used in situations where a strong, small base is required.
Thus, \[{\text{NaN}}{{\text{H}}_{\text{2}}}\] converts internal alkyne to terminal alkyne and alcoholic \[{\text{KOH}}\] converts terminal alkyne to internal alkyne.
Hence, the correct option is A.
Additional information:
We know that Sodium amide (\[{\text{NaN}}{{\text{H}}_{\text{2}}}\]) is a strong base and is used for deprotonation of weak acids and also for elimination reactions. Treatment of either geminal dihalide (two halogens on one carbon) or vicinal dihalides (halogens on adjacent carbons) with two equivalents of \[{\text{NaN}}{{\text{H}}_{\text{2}}}\] results in the formation of alkynes. The mechanism of this reaction can be shown as:

Note:
We should keep in mind the formation of terminal alkynes by use of this reaction mechanism. The acidity of terminal alkynes plays an important role in major product determination when dihalides undergo base induced elimination reactions. High electronegativity of the triple bond in terminal alkynes makes the molecule acidic. Therefore, one of the base molecules will pull off the terminal hydrogen instead of one of the halides like we desire to happen in this reaction. This implies that we would need three bases for every terminal haloalkane instead of two to obtain an alkyne.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




