
Intramolecular hydrogen bonding is found in:
A. salicylaldehyde
B. water
C. acetaldehyde
D. phenol
Answer
528.9k+ views
Hint: Hydrogen bonding is a dipole-dipole attraction between the molecules, but is not a covalent bond to a hydrogen atom. When the hydrogen is attached to a very electronegative element, then this type of bonding takes place. It is represented by a dotted line.
Complete step by step answer:
Hydrogen bond or H-bond is a partial intermolecular bond between a lone pair which is on the electron rich donor atom and the antibonding molecular orbital of a H atom. Hydrogen bonds are of two types: intramolecular hydrogen bond and intermolecular hydrogen bond. In intramolecular H-bonding, the bonds occur in the same molecule of a solution whereas, in intermolecular H-bonding, the bonds occur between two different molecules of a solution.
Now, let's look at the options.
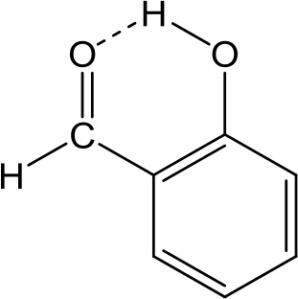
A. In salicylaldehyde, intramolecular H-bonds exist between the hydrogen atom from the hydroxy group and the oxygen atom from the aldehyde group.
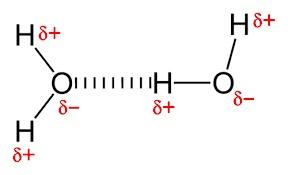
B. In water, the intermolecular H-bonding exists between the hydrogen atom from one molecule and the oxygen atom from another molecule.
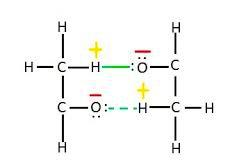
C. In acetaldehyde, the intermolecular H-bonding exists between the hydrogen atom from one molecule and the oxygen atom from another molecule.
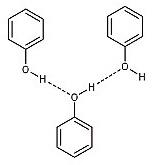
D. In phenol, the intermolecular H-bonding exists between the hydrogen atom from one molecule and the oxygen atom from another molecule.
Therefore, from the above diagrams, we can see that only salicylic acid has intramolecular hydrogen bonding.
Hence, the correct answer is option (a).
Note: Salicylic acid is a strong acid. The hydrogen bonding in salicylic acid lowers its free energy which makes salicylate a weaker conjugate base. And thus, salicylic acid is a strong acid.
Complete step by step answer:
Hydrogen bond or H-bond is a partial intermolecular bond between a lone pair which is on the electron rich donor atom and the antibonding molecular orbital of a H atom. Hydrogen bonds are of two types: intramolecular hydrogen bond and intermolecular hydrogen bond. In intramolecular H-bonding, the bonds occur in the same molecule of a solution whereas, in intermolecular H-bonding, the bonds occur between two different molecules of a solution.
Now, let's look at the options.
A. In salicylaldehyde, intramolecular H-bonds exist between the hydrogen atom from the hydroxy group and the oxygen atom from the aldehyde group.

B. In water, the intermolecular H-bonding exists between the hydrogen atom from one molecule and the oxygen atom from another molecule.

C. In acetaldehyde, the intermolecular H-bonding exists between the hydrogen atom from one molecule and the oxygen atom from another molecule.

D. In phenol, the intermolecular H-bonding exists between the hydrogen atom from one molecule and the oxygen atom from another molecule.

Therefore, from the above diagrams, we can see that only salicylic acid has intramolecular hydrogen bonding.
Hence, the correct answer is option (a).
Note: Salicylic acid is a strong acid. The hydrogen bonding in salicylic acid lowers its free energy which makes salicylate a weaker conjugate base. And thus, salicylic acid is a strong acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a labelled diagram of the human heart and label class 11 biology CBSE

What is 1s 2s 2p 3s 3p class 11 chemistry CBSE




