
Iodoform is formed on warming \[{{I}_{2}}\] and \[NaOH\] with:
Answer
517.2k+ views
Hint: We know that the reaction with iodine and sodium hydroxide is known as Haloform reaction and commonly known as Iodoform test. This test is known as the Iodoform test as iodoform is formed as a by-product. The basic condition for the iodoform test is given by the compounds which have methyl ketone.
Complete answer: First, we will understand the basic haloform reaction. According to haloform reaction, when a methyl ketone (even acetaldehyde) is reacted with halogen and that too in aqueous sodium hydroxide. During the reaction, the ketone gets oxidized to the sodium salt of acid with one carbon atom less than the ketone. At the same time, the haloform also gets formed. Therefore, this reaction is also considered as an Iodoform test. We can use this iodoform test to check which compound does not react.
So, when the haloform reaction is carried out with iodine, a yellow precipitate of iodine is formed or obtained. So, the formation of a yellow precipitate of iodoform is used to detect the presence. On adding iodine to caustic soda (alkali) hypoiodite is formed and a peculiar smell is of iodoform and pale yellow colour.
Following reaction with take place with \[{{C}_{2}}{{H}_{5}}OH.\]
\[{{C}_{2}}{{H}_{5}}OH+{{I}_{2}}+NaOH\to C{{H}_{3}}COONa+CH{{I}_{3}}\]
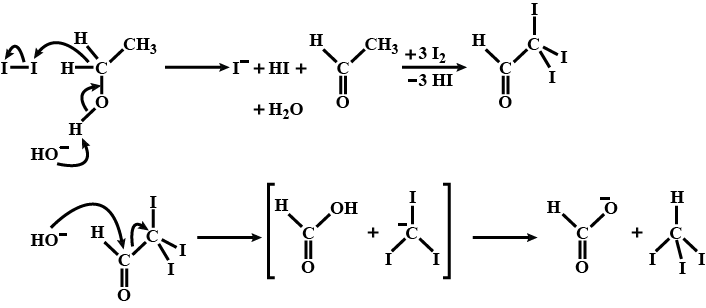
Therefore, Iodoform is formed on warming \[{{I}_{2}}\] and \[NaOH\] with \[{{C}_{2}}{{H}_{5}}OH.\]
Additional Information: Besides it is a main compound of soap and detergent. Other applications include water treatment, food textile, metal processing, mining, glass making etc. it is also widely used in laboratories for experimentation. Caustic soda is harmful. It can cause burns and permanent damage to the tissue which comes in direct contact.
Note:
Remember that the iodoform test is given by methyl ketones, acetaldehydes, ethanol, and compounds such as which are oxidized to appropriate carbonyl compounds under the conditions used for the reaction. The sodium hydroxide \[\left( \text{ }NaOH\text{ } \right)\] used in the reaction should be cold and dilute. Reaction will not take place if \[NaOH\] is hot and concentrated.
Complete answer: First, we will understand the basic haloform reaction. According to haloform reaction, when a methyl ketone (even acetaldehyde) is reacted with halogen and that too in aqueous sodium hydroxide. During the reaction, the ketone gets oxidized to the sodium salt of acid with one carbon atom less than the ketone. At the same time, the haloform also gets formed. Therefore, this reaction is also considered as an Iodoform test. We can use this iodoform test to check which compound does not react.
So, when the haloform reaction is carried out with iodine, a yellow precipitate of iodine is formed or obtained. So, the formation of a yellow precipitate of iodoform is used to detect the presence. On adding iodine to caustic soda (alkali) hypoiodite is formed and a peculiar smell is of iodoform and pale yellow colour.
Following reaction with take place with \[{{C}_{2}}{{H}_{5}}OH.\]
\[{{C}_{2}}{{H}_{5}}OH+{{I}_{2}}+NaOH\to C{{H}_{3}}COONa+CH{{I}_{3}}\]

Therefore, Iodoform is formed on warming \[{{I}_{2}}\] and \[NaOH\] with \[{{C}_{2}}{{H}_{5}}OH.\]
Additional Information: Besides it is a main compound of soap and detergent. Other applications include water treatment, food textile, metal processing, mining, glass making etc. it is also widely used in laboratories for experimentation. Caustic soda is harmful. It can cause burns and permanent damage to the tissue which comes in direct contact.
Note:
Remember that the iodoform test is given by methyl ketones, acetaldehydes, ethanol, and compounds such as which are oxidized to appropriate carbonyl compounds under the conditions used for the reaction. The sodium hydroxide \[\left( \text{ }NaOH\text{ } \right)\] used in the reaction should be cold and dilute. Reaction will not take place if \[NaOH\] is hot and concentrated.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




