
How many isomers of ${C_4}{H_8}C{l_2}$ are possible?
Answer
555.3k+ views
Hint: Try to make different structures in your notebook firstly by putting all carbons in a straight line and swapping the positions of chlorine atoms count the number of isomers possible with it and then move one carbon atom to make a secondary position. This also places chlorine atoms in different arrangements.
Complete step-by-step answer:
For counting possible structural isomers there is formula available but drawing different structures helps a lot because using the drawing you can easily see the difference in two structures. Firstly, if I put all four carbon atoms in a straight line, then I can put both chlorine atoms with carbon no. $1$ or I can attach both with carbon number $2$ in these structure you get,
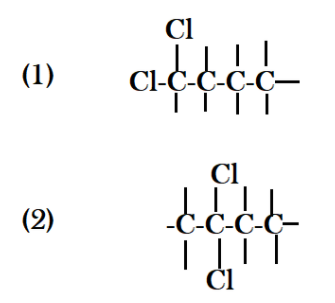
Now if we put both chlorines in different positions, I mean if I put one chlorine at carbon no. $1$ and other at carbon no.$2$, similarly if I take second chlorine from carbon no. $2$ and place it with carbon $3$ and $4$ I get three different structure.
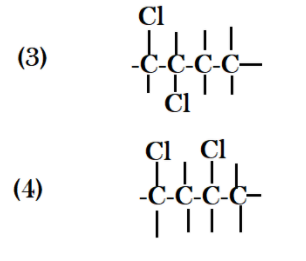
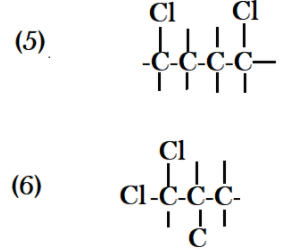
Now if I make a branched chain by connecting carbon no. $4$ with carbon no. $2$ then I also get a different structure. Let’s start placing two chlorine atoms with the same carbon and in different structures with two different carbon. When we place chlorine with two different carbons there are two possibilities that in first one, one chlorine gets attached to carbon no. $1$ and carbon no. $2$ and in second type chlorine atoms can get attached with carbon $1$ and carbon $3$ .
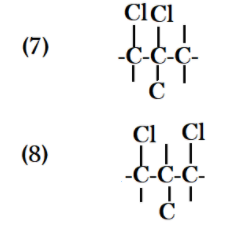
Count all the possible structures that you have drawn and you get an idea that you had made eight different types of structures with formula ${C_4}{H_8}C{l_2}$ .
Note: There are some cases in which you made diagrams which are exactly the same, it means it can happen that two diagrams become the same so cut them and count them one. All the valences that are leaved in the diagrams are the positions of all hydrogen atoms. Always count the number of atoms of chlorine, carbon and hydrogen to confirm that you are satisfying the structural possibilities of the formula given.
Complete step-by-step answer:
For counting possible structural isomers there is formula available but drawing different structures helps a lot because using the drawing you can easily see the difference in two structures. Firstly, if I put all four carbon atoms in a straight line, then I can put both chlorine atoms with carbon no. $1$ or I can attach both with carbon number $2$ in these structure you get,
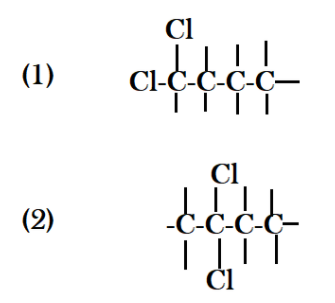
Now if we put both chlorines in different positions, I mean if I put one chlorine at carbon no. $1$ and other at carbon no.$2$, similarly if I take second chlorine from carbon no. $2$ and place it with carbon $3$ and $4$ I get three different structure.
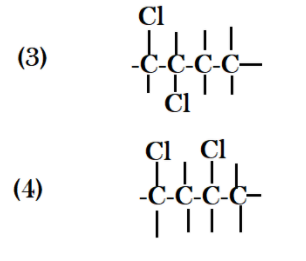
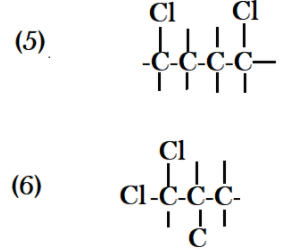
Now if I make a branched chain by connecting carbon no. $4$ with carbon no. $2$ then I also get a different structure. Let’s start placing two chlorine atoms with the same carbon and in different structures with two different carbon. When we place chlorine with two different carbons there are two possibilities that in first one, one chlorine gets attached to carbon no. $1$ and carbon no. $2$ and in second type chlorine atoms can get attached with carbon $1$ and carbon $3$ .
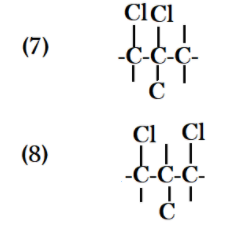
Count all the possible structures that you have drawn and you get an idea that you had made eight different types of structures with formula ${C_4}{H_8}C{l_2}$ .
Note: There are some cases in which you made diagrams which are exactly the same, it means it can happen that two diagrams become the same so cut them and count them one. All the valences that are leaved in the diagrams are the positions of all hydrogen atoms. Always count the number of atoms of chlorine, carbon and hydrogen to confirm that you are satisfying the structural possibilities of the formula given.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




