
Iso-propyl bromide on Wurtz reaction gives:
(A) Hexane
(B) Propane
(C) 2,3-dimethyl butane
(D) Neo-hexane
Answer
529.5k+ views
Hint:
The structure of isopropyl group is
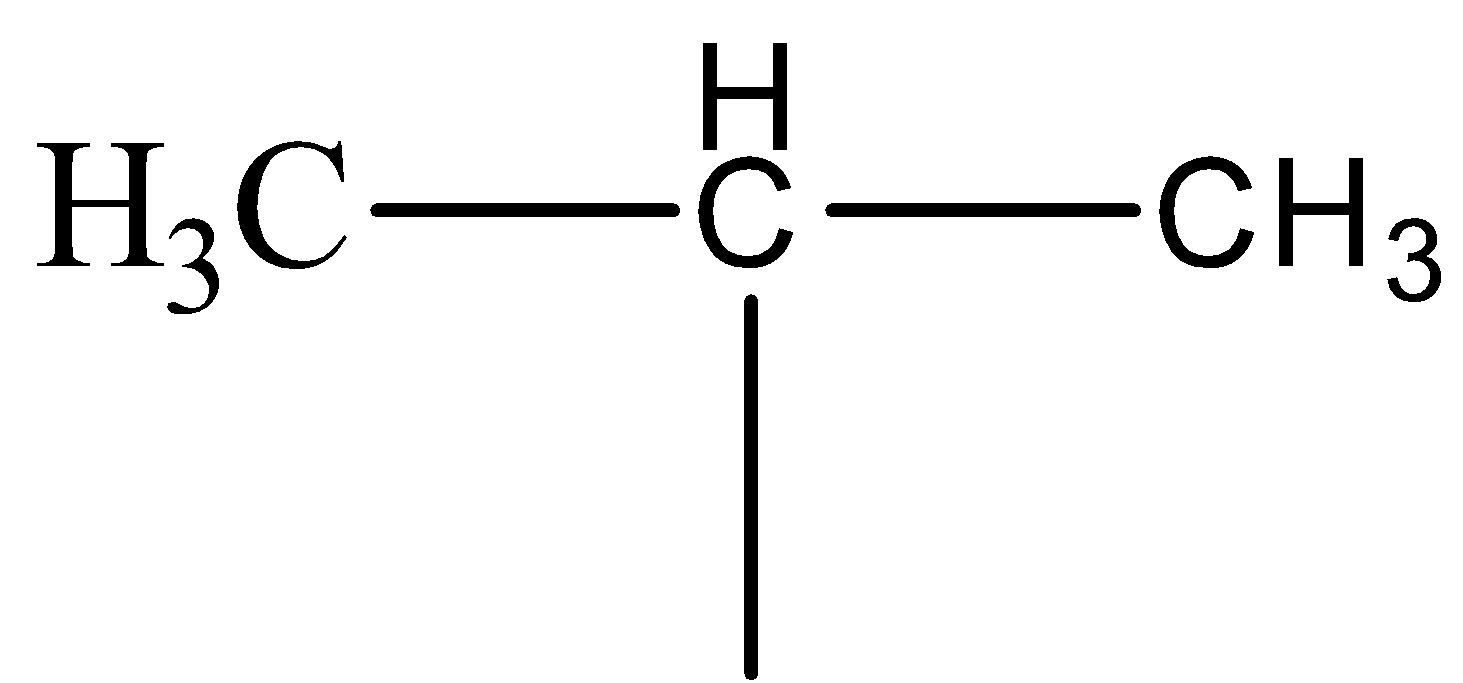
Wurtz reaction gives Sodium halide as a side product in the reaction.
Complete step by step answer:
Let’s first see what happens in the Wurtz reaction.
In Wurtz reaction, two alkyl halides couple in presence of sodium metal and give alkane and sodium halide in ether solvent. The reaction can be written as,
\[2R - X\xrightarrow[{Ether}]{{Na}}R - R + NaX\]
Now let’s see the structure of isopropyl bromide.
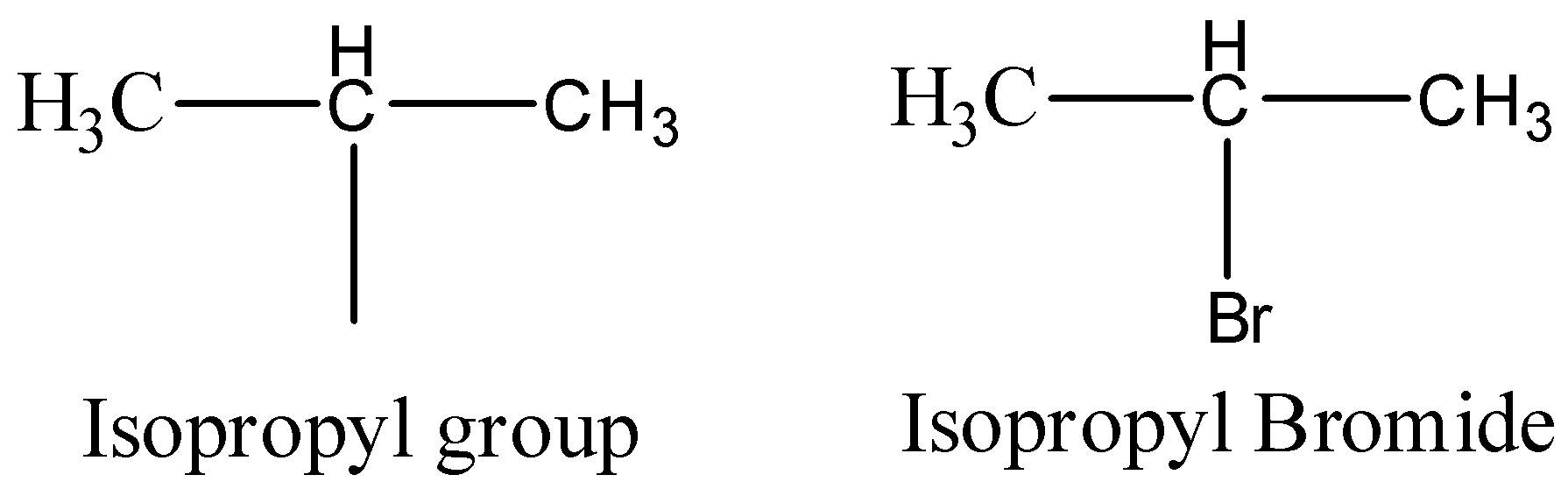
So, if isopropyl bromide will be allowed to react with Sodium metal in ether solvent then, sodium metal will act as an reducing agent and will form a salt sodium bromide. Then the two isopropyl groups will combine and will give an alkane as a final product. So, the reaction can be written as below.
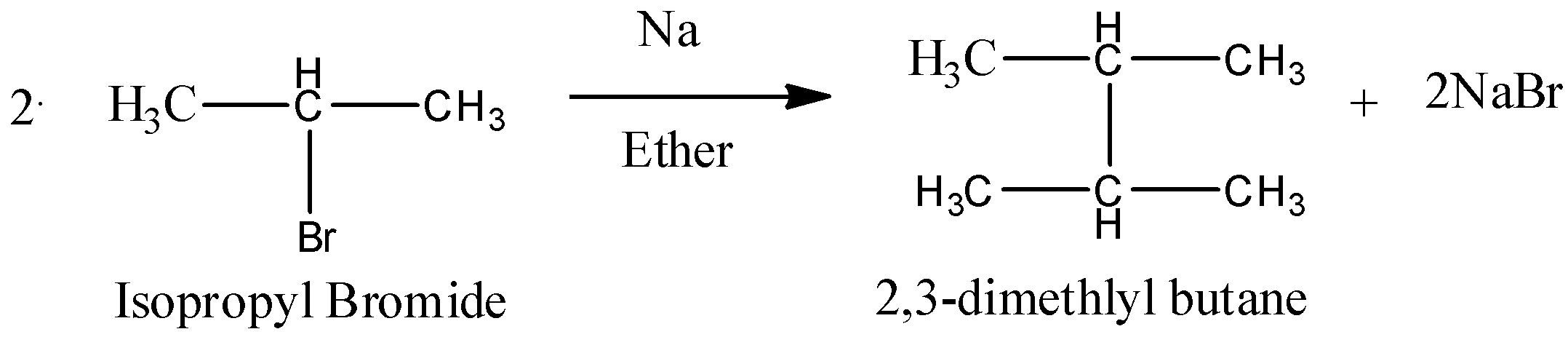
So, we can conclude that 2,3-dimethylbutane is the final product of the reaction.
Therefore the correct answer is (C) 2,3-dimethyl butane.
Additional Information:
See some of the other similar looking reactions as Wurtz reaction to avoid confusion.
Fitting reaction:
\[2Ar - X\xrightarrow[{Ether}]{{Na}}Ar - Ar + NaX\]
Here we can see that two aromatic halides react with sodium metal in ether to give coupled product and sodium halides as a side product.
Wurtz-Fittig reaction:
\[R - X + Ar - X\xrightarrow[{Ether}]{{Na}}Ar - R + NaX\]
Here we can see that one alkyl halide and one aryl halides are used as a starting material. They combine in presence of sodium metal and ether as solvent to give aromatic alkanes and sodium halide.
Note:
Do not get confused by two other same looking reactions like Wurtz reaction, which is Fittig reaction and Wurtz –Fittig reaction. Remember that in the isopropyl group, the substituent group will get bonded with the \[{2^{nd}}\] carbon of the group, so do not get confused with the n-propyl group.
The structure of isopropyl group is

Wurtz reaction gives Sodium halide as a side product in the reaction.
Complete step by step answer:
Let’s first see what happens in the Wurtz reaction.
In Wurtz reaction, two alkyl halides couple in presence of sodium metal and give alkane and sodium halide in ether solvent. The reaction can be written as,
\[2R - X\xrightarrow[{Ether}]{{Na}}R - R + NaX\]
Now let’s see the structure of isopropyl bromide.
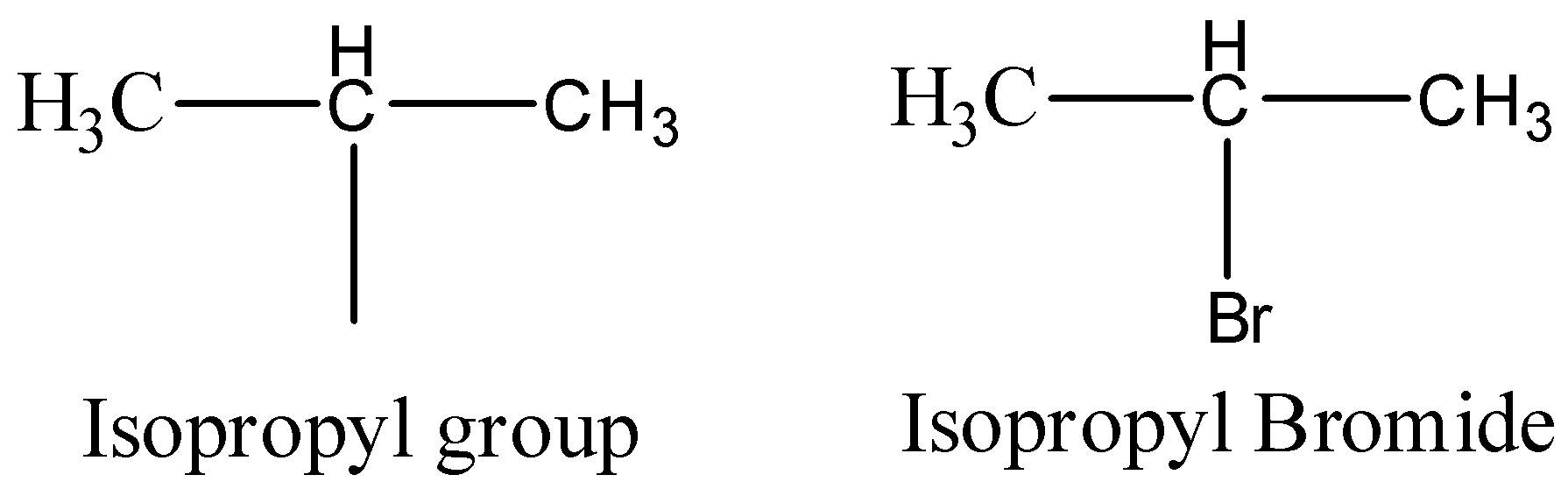
So, if isopropyl bromide will be allowed to react with Sodium metal in ether solvent then, sodium metal will act as an reducing agent and will form a salt sodium bromide. Then the two isopropyl groups will combine and will give an alkane as a final product. So, the reaction can be written as below.

So, we can conclude that 2,3-dimethylbutane is the final product of the reaction.
Therefore the correct answer is (C) 2,3-dimethyl butane.
Additional Information:
See some of the other similar looking reactions as Wurtz reaction to avoid confusion.
Fitting reaction:
\[2Ar - X\xrightarrow[{Ether}]{{Na}}Ar - Ar + NaX\]
Here we can see that two aromatic halides react with sodium metal in ether to give coupled product and sodium halides as a side product.
Wurtz-Fittig reaction:
\[R - X + Ar - X\xrightarrow[{Ether}]{{Na}}Ar - R + NaX\]
Here we can see that one alkyl halide and one aryl halides are used as a starting material. They combine in presence of sodium metal and ether as solvent to give aromatic alkanes and sodium halide.
Note:
Do not get confused by two other same looking reactions like Wurtz reaction, which is Fittig reaction and Wurtz –Fittig reaction. Remember that in the isopropyl group, the substituent group will get bonded with the \[{2^{nd}}\] carbon of the group, so do not get confused with the n-propyl group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




