
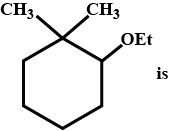
IUPAC name of given compound is:
Option
A.1,1 dimethyl 2-ethoxy cylcohexane
B.2-ethoxy 1,1 dimethyl cylcohexane
C.1,2 dimethyl 2-ethoxy cylcohexane
D.2,2 dimethyl 1-ethoxy cylcohexane

Answer
519k+ views
Hint: In the very least, a logical nomenclature scheme can accomplish two goals. To begin, it should show how a compound's carbon atoms are bound together in a specific lattice of chains and rings. Second, any functional groups present in the compound should be identified and located. Since hydrogen is such a basic part of organic compounds, its number and position can usually be deduced from the tetravalency of carbon, and it isn't necessary to specify it.
Complete answer:
The IUPAC nomenclature scheme is a collection of conceptual rules developed and used by organic chemists to avoid the problems that arbitrary nomenclature can create. Knowing these laws and being given a structural formula, one should be able to write a distinct name for each compound. A structural formula should be able to be written given an IUPAC tag. An IUPAC name would, in general, have three characteristics:
• A root or basis in a molecular structure that denotes a main chain or ring of carbon atoms.
• A suffix or other element(s) indicating the presence of functional classes in the compound.
• Names of substituent groups that complete the molecular structure but are not hydrogen.
IUPAC Rules for Cycloalkane Nomenclature
1. The ring provides the root name for a monosubstituted cycloalkane, and the substituent group is called normal. It is not appropriate to have a position number.
2. The ring will be named as a substituent group on an alkane if the alkyl substituent is large and/or complex.
3. If the ring contains two separate substituents, they are classified in alphabetical order, with the first cited substituent being attributed to carbon #1. After that, the ring carbons are numbered in a direction (clockwise or counter-clockwise) that gives the second substituent the lowest possible position number.
4. If the ring contains several substituents, they are classified in alphabetical order. The substituents are given position numbers such that one is at carbon #1 and the others have the lowest possible numbers, counting in either a clockwise or counter-clockwise direction.
5. The name is put together, with groups listed alphabetically and a position number assigned to each group (if there are two or more). When alphabetizing, the prefixes di, tri, tetra, and others, which are used to label many classes of the same kind, are ignored.
Hence using the above 5 rules, we name the compound
Root – Cyclohexane
Suffix – di methyl in 1st position and ethoxy in 2nd position
i.e, 2-ethoxy 1,1- dimethyl cylcohexane
Hence option B is correct.
Note:
The name is put together, with groups listed alphabetically and a position number assigned to each group (if there are two or more). When alphabetizing, the prefixes di, tri, tetra, and others, which are used to label many classes of the same kind, are ignored.
Complete answer:
The IUPAC nomenclature scheme is a collection of conceptual rules developed and used by organic chemists to avoid the problems that arbitrary nomenclature can create. Knowing these laws and being given a structural formula, one should be able to write a distinct name for each compound. A structural formula should be able to be written given an IUPAC tag. An IUPAC name would, in general, have three characteristics:
• A root or basis in a molecular structure that denotes a main chain or ring of carbon atoms.
• A suffix or other element(s) indicating the presence of functional classes in the compound.
• Names of substituent groups that complete the molecular structure but are not hydrogen.
IUPAC Rules for Cycloalkane Nomenclature
1. The ring provides the root name for a monosubstituted cycloalkane, and the substituent group is called normal. It is not appropriate to have a position number.
2. The ring will be named as a substituent group on an alkane if the alkyl substituent is large and/or complex.
3. If the ring contains two separate substituents, they are classified in alphabetical order, with the first cited substituent being attributed to carbon #1. After that, the ring carbons are numbered in a direction (clockwise or counter-clockwise) that gives the second substituent the lowest possible position number.
4. If the ring contains several substituents, they are classified in alphabetical order. The substituents are given position numbers such that one is at carbon #1 and the others have the lowest possible numbers, counting in either a clockwise or counter-clockwise direction.
5. The name is put together, with groups listed alphabetically and a position number assigned to each group (if there are two or more). When alphabetizing, the prefixes di, tri, tetra, and others, which are used to label many classes of the same kind, are ignored.
Hence using the above 5 rules, we name the compound
Root – Cyclohexane
Suffix – di methyl in 1st position and ethoxy in 2nd position
i.e, 2-ethoxy 1,1- dimethyl cylcohexane
Hence option B is correct.
Note:
The name is put together, with groups listed alphabetically and a position number assigned to each group (if there are two or more). When alphabetizing, the prefixes di, tri, tetra, and others, which are used to label many classes of the same kind, are ignored.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




