
IUPAC name of t-butyl alcohol.
Answer
600.3k+ views
Hint: Steps to write the IUPAC name of a compound:
1. Identify the functional group (it can be alcohol, aldehyde, ether etc.).
2. Find the longest carbon chain.
3. Number of carbon atoms present in the longest chain.
4. If there is any branched group, identify their position and name them.
5. Combine the element names in a single word.
Complete step by step solution:
Now in tertiary butyl alcohol, there are a total of 4 carbon atoms with an alcohol group attached to the carbon atom.
The chemical formula for tertiary butyl alcohol is \[{{(C{{H}_{3}})}_{3}}COH\], with three methyl groups and an alcohol group connected to the same carbon atom.
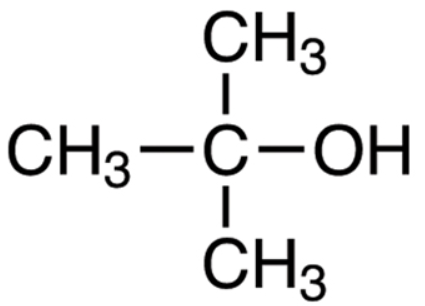
We can see the longest chain has 3 carbon atoms, so let's consider the IUPAC name will have propane in it.
Now, the second carbon in the propane chain has two branched groups, one is methyl and the other is an alcohol group.
We know an alcohol derived from an alkane will substitute the -e from alkane to -ol.
We name the compounds in an alphabetical manner, so, methyl will be written first and as it is present on the second carbon. We will write number 2 before methyl, same as we will do with the alcohol group.
Therefore, the IUPAC name of tert-butyl alcohol will be 2-methylpropan-2-ol.
From the above statements we can conclude that the IUPAC name for tert-butyl alcohol will be 2-methylpropan-2-ol.
Note: Tert-butyl alcohol (t- or tert- suffix given for tertiary) is a colorless solid which melts into oily liquid with a sharp alcohol or camphor odor at room temperature. It is miscible with water, producing an irritating vapor.
1. Identify the functional group (it can be alcohol, aldehyde, ether etc.).
2. Find the longest carbon chain.
3. Number of carbon atoms present in the longest chain.
4. If there is any branched group, identify their position and name them.
5. Combine the element names in a single word.
Complete step by step solution:
Now in tertiary butyl alcohol, there are a total of 4 carbon atoms with an alcohol group attached to the carbon atom.
The chemical formula for tertiary butyl alcohol is \[{{(C{{H}_{3}})}_{3}}COH\], with three methyl groups and an alcohol group connected to the same carbon atom.

We can see the longest chain has 3 carbon atoms, so let's consider the IUPAC name will have propane in it.
Now, the second carbon in the propane chain has two branched groups, one is methyl and the other is an alcohol group.
We know an alcohol derived from an alkane will substitute the -e from alkane to -ol.
We name the compounds in an alphabetical manner, so, methyl will be written first and as it is present on the second carbon. We will write number 2 before methyl, same as we will do with the alcohol group.
Therefore, the IUPAC name of tert-butyl alcohol will be 2-methylpropan-2-ol.
From the above statements we can conclude that the IUPAC name for tert-butyl alcohol will be 2-methylpropan-2-ol.
Note: Tert-butyl alcohol (t- or tert- suffix given for tertiary) is a colorless solid which melts into oily liquid with a sharp alcohol or camphor odor at room temperature. It is miscible with water, producing an irritating vapor.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




