
What is the IUPAC name of the given compound?
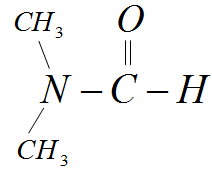
A) N,N-dimethylmethanal
B) N,N-dimethylmethanamide
C) N,N-dimethylcarbaldehyde
D) None of the above
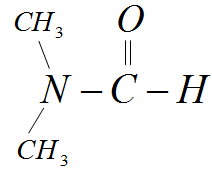
Answer
564.9k+ views
Hint: The molecular formula for the given structure is ${C_3}{H_7}NO$ . It is a tertiary amide consisting of one carboxyl group attached to Nitrogen. The other two bonds of Nitrogen are filled with two methyl groups. According to the IUPAC functional group priority table, the priority must be given to amide in this compound.
Complete step by step solution:
- We can use the IUPAC nomenclature rules to name the given compound.
-We follow the IUPAC functional group priority table and give the first priority to tertiary amide. Therefore the suffix for the IUPAC name would be ‘amide’
-This amide group consists of two methyl groups so we can write the prefix for the IUPAC name as ‘N,N-dimethyl’. There is only one bond left which is a carbonyl group. By combining all these, we can write the IUPAC name as N,N-dimethylmethanamide.
Therefore the IUPAC name of the given structure is N,N-dimethylmethanamide i.e. option B.
Additional information: N,N-dimethylmethanamide is also known as N,N-dimethylformamide. This compound is a white liquid and has a faint fishy odor. This compound is used in the production of films, fibers, surface coatings etc. Overexposure to this compound leads to lung damage in humans and animals.
Note:Given compound is a tertiary amide group with nitrogen attached to two methyl groups and one carbonyl group. Since this compound consists of two functional groups, we use the IUPAC rules in order to prioritize one group over another which becomes the suffix of the IUPAC name.
Complete step by step solution:
- We can use the IUPAC nomenclature rules to name the given compound.
-We follow the IUPAC functional group priority table and give the first priority to tertiary amide. Therefore the suffix for the IUPAC name would be ‘amide’
-This amide group consists of two methyl groups so we can write the prefix for the IUPAC name as ‘N,N-dimethyl’. There is only one bond left which is a carbonyl group. By combining all these, we can write the IUPAC name as N,N-dimethylmethanamide.
Therefore the IUPAC name of the given structure is N,N-dimethylmethanamide i.e. option B.
Additional information: N,N-dimethylmethanamide is also known as N,N-dimethylformamide. This compound is a white liquid and has a faint fishy odor. This compound is used in the production of films, fibers, surface coatings etc. Overexposure to this compound leads to lung damage in humans and animals.
Note:Given compound is a tertiary amide group with nitrogen attached to two methyl groups and one carbonyl group. Since this compound consists of two functional groups, we use the IUPAC rules in order to prioritize one group over another which becomes the suffix of the IUPAC name.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




