
How many ketones are possible with the molecular formula\[{C_6}{H_{12}}O\] ?
A) \[5\]
B) \[6\]
C) \[7\]
D) \[8\]
Answer
558.9k+ views
Hint: To answer the question we need to know how we can draw structures from a given molecular formula. Utilizing evenness, and the way that it should be a ketone, the carbonyl group can just move between carbons \[2\] and\[3\]. That makes for the initial two isomers. Two additional isomers include moving the terminal methyl bunch up one to carbon\[4\]. Two additional isomers include moving the terminal methyl bunch up one to carbon\[4\]. The fifth and 6th isomers are enantiomers, the highest point of which is \[R\] and the lower part of which is\[S\]. The thought was to move the methyl bunch up one more to carbon\[3\], making a sec-butyl bunch off of\[{(C{H_3})_2}CO\]. The seventh isomer was essentially to make a tert-butyl bunch hanging off of\[{(C{H_3})_2}CO\].
Complete step-by-step answer:
The simplest complex ketone is\[C{H_3} - C( = O) - C{H_3}\]. Its atomic formula is\[{C_3}{H_6}O\]. From this formula, we can say that for "\[n\]" carbon iotas we need "\[2n\]" hydrogen molecules and an oxygen particle. Henceforth the overall recipe of the ketone is\[{C_n}{H_{2n}}O\].
Keto group contains a carboxyl gathering which has two alkyl bunches connected to it each on one or the other side. Primary formula: $R - {C = O} - {R^1}$. Aldehydes and ketones are natural mixes that join a carbonyl functional group,\[C = O\].
We can write seven ketone structures with the molecular formula\[{C_6}{H_{12}}O\].
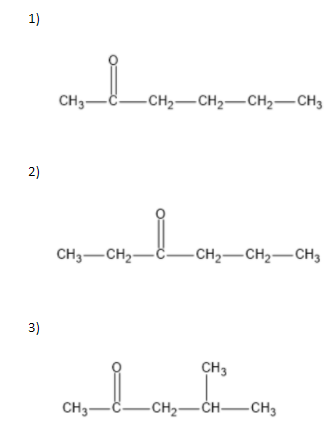
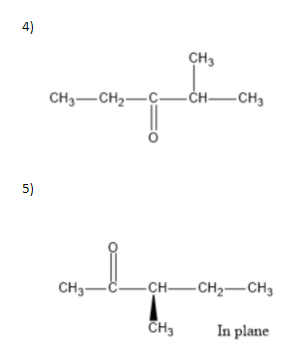
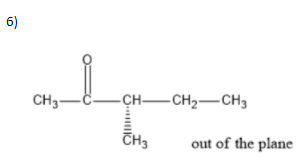
7)
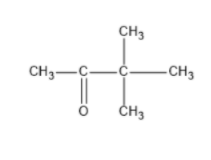
Note: We know that a carbonyl group can only exist at spots that give a \[s{p^2}\] hybridization, so there is no structure for the carbonyl on carbon \[3\] when carbon \[3\] has a tert-butyl group. Similarly, there is no structure for which the carbonyl is on carbon \[3\] while the sec-butyl is also on carbon\[3\].
Complete step-by-step answer:
The simplest complex ketone is\[C{H_3} - C( = O) - C{H_3}\]. Its atomic formula is\[{C_3}{H_6}O\]. From this formula, we can say that for "\[n\]" carbon iotas we need "\[2n\]" hydrogen molecules and an oxygen particle. Henceforth the overall recipe of the ketone is\[{C_n}{H_{2n}}O\].
Keto group contains a carboxyl gathering which has two alkyl bunches connected to it each on one or the other side. Primary formula: $R - {C = O} - {R^1}$. Aldehydes and ketones are natural mixes that join a carbonyl functional group,\[C = O\].
We can write seven ketone structures with the molecular formula\[{C_6}{H_{12}}O\].



7)

Note: We know that a carbonyl group can only exist at spots that give a \[s{p^2}\] hybridization, so there is no structure for the carbonyl on carbon \[3\] when carbon \[3\] has a tert-butyl group. Similarly, there is no structure for which the carbonyl is on carbon \[3\] while the sec-butyl is also on carbon\[3\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




